Subatomic particles located in the nucleus.
Neutrons and protons.
2 examples of physical properties.
color, mass, density, texture,
12ft in inches
144inches
Empirical formula of C9H21O3
C3H7O
The amount of a mole.
6.022x1023
Atomic number of Tungsten.
74
2 examples of chemical properties.
Flammability, rustability, reactivity, toxicity, ect.
Number of miles in 2yds
0.00114miles or 1.14x10-3miles
The molar mass of CaCl2
110g/mol
CO2 and O2 stirred together creates a BLANK of a BLANK and a BLANK.
Mixture of a compound and element.
AZX isotope of p+= 24 n0= 27 e-= 22
5124Cr2+
This is an example of a....
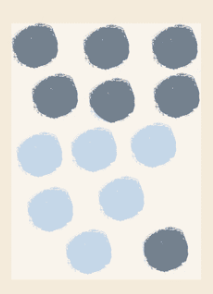
Heterogeneous mixture
5304mL into kL
0.005304kL or 5.304x10-3kL
Percent composition of potassium in KCl.
52.44%
The type of particle to have an even number of all subatomic particles.
An atom
How many protons, neutrons, and electrons are present?

p+=4 n0=5 e-=6
List an element, compound, and a homogenous mixture.
Answers will vary
Example: Sodium, Sodium chloride, and salt water.
The volume of 20g of iron, given if the density is 7.874 g/cm³
2.54cm3
34.6amu
The average atomic mass of an element whos isotopes are 78amu, at 25%, 79amu, at 20%, and 80amu, at 55%.
79.3amu
The mass of 1 electron.
0.0005amu
The difference between a chemical and physical change.
Physical doesn't change the chemical composition but a chemical change rearranges the molecular structure.
2mol into dozens
1.004x1023dozens
The molecular(regular) formula of given a compound with an empirical formula of PbO2 and a molar mass of 1,673g/mol.
Pb7O14
What subject does Mr. Ryfiak's husband teach?
Spanish