Is a melting popsicle a chemical or physical change?
Physical Change
During our bath bomb experiments, the gas produced did not extinguish the flame. True or False
False
Which is denser Helium (He) or Nitrogen (N)?
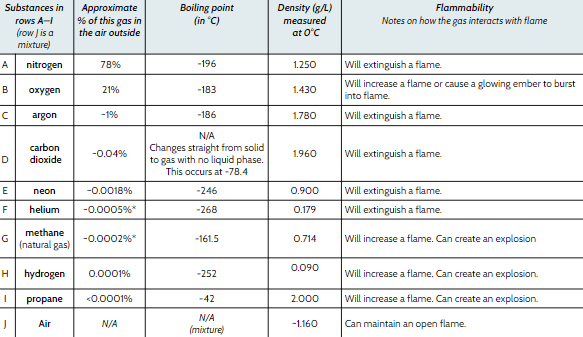
Nitrogen
What is the formula for density?
D=M/V
Which unit would you use for the weight of an elephant?
Kilograms
Is burning wood a chemical or physical change?
Chemical Change
A boiling pot of water with its lid off is an example of what type of system?
Open System
What is the flammability of Methane?
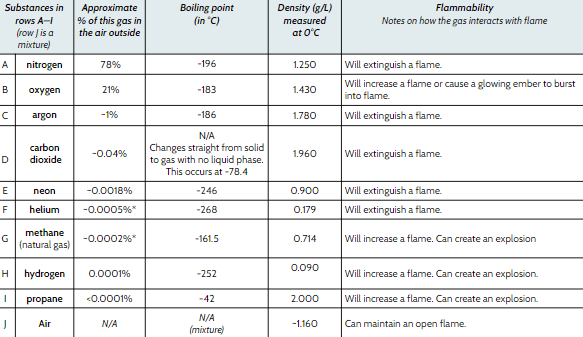
Increase a flame
You have a rock with a volume of 15 cm3 and a mass of 45 g. What is its density?
3 g/cm3
Draw an example of a closed system
First and best wins
Is digesting food a chemical or physical change?
Chemical Change
A metal thermos full of coffee with a lid on a bench at a hockey game is an example of what type of system?
Which gas makes up the largest percentage of air?
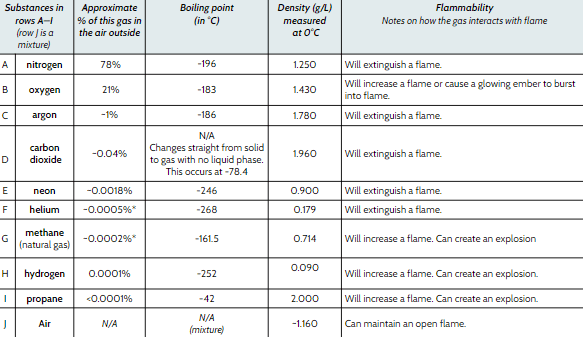
Nitrogen
Solve for density of an unknown substance
Mass = 200 grams
Volume = 25 cm3
8 g/cm3
Which unit would you use to measure the volume of a liquid in a teaspoon of vanilla? 
Milliliters (mL)
Is a nail rusting a chemical or physical change?
chemical change
We placed the bath bomb ingredients into a test tube and placed the ingredients inside the soda bottle. Then we caused the reaction and weighed the bottle closed and open.
In that experiment, we learned that?
That the gas is not a new matter
Which gas has the lowest boiling point? 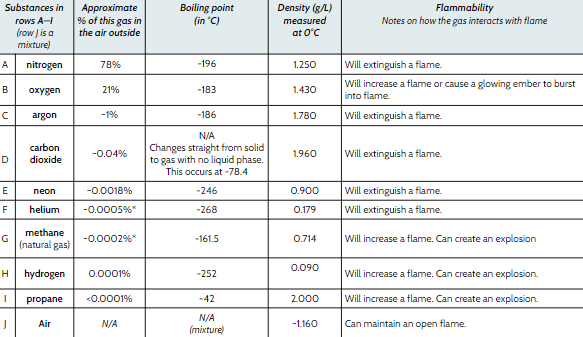
Helium (He)
A golden-colored cube is handed to you. The person wants you to buy it for $100, saying that is a gold nugget. You pull out your old geology text and look up gold in the mineral table, and read that its density is 19.3 g/cm3. You measure the cube and find that it is 2 cm on each side, and weighs 40 g. What is its density? Is it gold? Should you buy it?
5.0 g/cm3, No, and No.
What is the difference between a qualitative and a quantitative observation?
Qualitative describes the quality of an object such as color or scent.
Quantitative describes a quantity such as mass of an object of the height of an object expressed as a number.
Dissolving sugar and Kool-Aid mix into water is an example of chemical or physical change.
Physical Change
The fact that NaHCO3 + C₆H₈O₇ + H2O make CO2 and not Argon or Nitrogen gas is an example of which law of science?
The Law of Conservation of Mass
Carbon dioxide is ________ than air and will _________ a flame.
denser, extinguish
You decide you want to carry a boulder home from the beach. It is 30 centimeters on each side, and so has a volume of 27,000 cm3. It is made of granite, which has a typical density of 2.8 g/cm3. How much will this boulder weigh?
1 pound = 453.6 grams
The result is that the mass is 75,600 grams. That is over 165 pounds.
List out and write the definitions of Control, Dependent, and Independent variables.
Control - do not change
Dependent - responds to change
Independent - the one you do change