Which of the following is a monosaccharide?
A) Sucrose B) Starch C) Glucose D) Cellulose
C) Glucose
Which of the following is NOT a lipid?
A) Phospholipid
B) Steroid
C) Triglyceride
D) Hemoglobin
D) Hemoglobin
Proteins are polymers of
A) Nucleotides
B) Amino acids
C) Fatty acids
D) Monosaccharides
B) Amino acids
DNA and RNA are polymers of
A) Amino acids
B) Monosaccharides
C) Nucleotides
D) Fatty acids
C) Nucleotides
Water molecules are polar because:
A) Oxygen and hydrogen share electrons equally.
B) Oxygen has a higher electronegativity than hydrogen, creating partial charges.
C) Water molecules form only nonpolar covalent bonds.
D) Hydrogen atoms carry a full positive charge.
B
Explanation:
Oxygen attracts shared electrons more strongly than hydrogen, creating a partial negative charge (δ-) near oxygen and partial positive charges (δ+) near the hydrogens. This polarity leads to many of water’s unique properties.
Which polysaccharide is used for energy storage in animals?
A) Cellulose
B) Glycogen
C) Chitin
D) Starch
B) Glycogen
What part of a phospholipid is hydrophilic?
A) Fatty acid tail
B) Phosphate head
C) Glycerol backbone
D) Hydrocarbon chain
B) Phosphate head
Which bond is found between amino acids in a protein? A) Glycosidic
B) Ester
C) Peptide
D) Hydrogen
C) Peptide
Which of the following is found in DNA but not RNA?
A) Uracil
B) Thymine
C) Ribose
D) Phosphate group
B) Thymine
A water droplet sticking to a spider web is primarily due to:
A) Cohesion between water molecules.
B) Ionic bonds between water and the web.
C) Adhesion between water molecules and the silk.
D) Hydrogen bonds within the silk fibers.
Correct Answer: C
Explanation:
Adhesion is the attraction between different molecules. Here, water molecules form hydrogen bonds with the silk proteins, causing the water droplet to cling to the web.
Which statement about carbohydrates is correct?
A) They are composed of amino acids
B) They contain C, H, O typically in a 1:2:1 ratio
C) They always form rings
D) They store genetic information
B) They contain C, H, O typically in a 1:2:1 ratio
Unsaturated fats differ from saturated fats because they
A) have fewer carbon atoms
B) have double bonds in the hydrocarbon chain
C) have no glycerol molecule
D) are only found in animals
B) have double bonds in the hydrocarbon chain
Enzymes speed up reactions by
A) Increasing activation energy
B) Decreasing activation energy
C) Adding energy to products
D) Acting as reactants
A) Increasing activation energy
Which type of bond holds the two strands of DNA together?
A) Covalent
B) Ionic
C) Hydrogen
D) Peptide
C) Hydrogen
Which property of water helps stabilize organisms’ internal temperatures and Earth’s climate?
A) Low specific heat capacity
B) High specific heat capacity
C) Low surface tension
D) High density when frozen
Correct Answer: B
Explanation:
Water has a high specific heat capacity, meaning it resists temperature changes by absorbing or releasing large amounts of heat without drastically changing its own temperature.
Which carbohydrate is a structural component in plant cell walls?
A)Glycogen
B) Starch
C) Cellulose
D) Glucose
C) Cellulose
Cholesterol is important for
A) Cell membrane structure
B) Making polysaccharides
C) Providing immediate energy
D) Catalyzing reactions
A) Cell membrane structure
Explain how temperature can denature a protein.
Temperature denatures proteins by:
breaks the non-covalent bonds and hydrophobic interactions
disrupts the protein's secondary, tertiary, and quaternary structures, leading to a loss of its functional shape while leaving the primary amino acid sequence intact.
Which statement about nucleic acids is true?
A) RNA is double-stranded
B) DNA contains ribose
C) DNA is the only nucleic acid that can carry genetic information
D) RNA can act as an enzyme
D) RNA can act as an enzyme
Question 4: Ice Density
Ice floats on liquid water because:
A) The hydrogen bonds in ice are weaker than in liquid water.
B) Ice molecules are more tightly packed than water molecules.
C) The crystal structure of ice spaces molecules farther apart, lowering its density.
D) Ice is less polar than liquid water.
Correct Answer: C
Explanation:
As water freezes, hydrogen bonds lock molecules into a lattice, increasing the distance between them and making ice less dense than liquid water.
Identify the molecule shown in the diagram
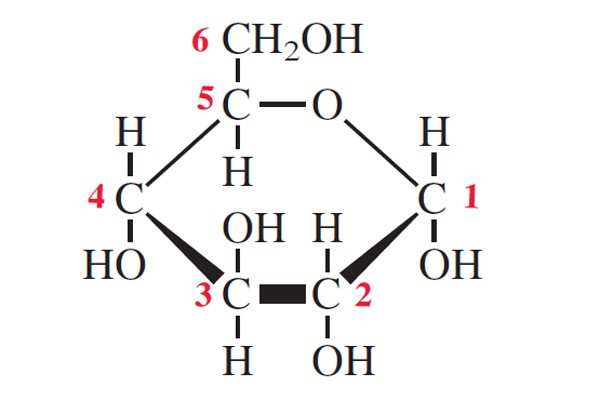
Glucose
Name the hydrophobic component in the phospholipid layer.
Fatty Acid Chains/Tails
Which component of an enzyme's structure determines its specificity?
Active Site
Describe one structural difference between DNA and RNA and explain its significance.
DNA is double-stranded while RNA is single-stranded.
The significance of this difference is that the double helix of DNA provides high stability, making it suitable for its role as long-term genetic information storage, while the single-stranded nature of RNA makes it more flexible and reactive, allowing it to perform diverse temporary functions, such as copying genetic instructions and aiding in protein synthesis.
Water is considered the “universal solvent” because:
A) It can dissolve any substance.
B) It is nonpolar and interacts equally with all molecules.
C) Its polarity allows it to dissolve many ionic and polar substances.
D) It forms covalent bonds with solutes.
Correct Answer: C
Explanation:
The polarity of water allows it to surround and separate ions or polar molecules, making it excellent at dissolving substances like salts and sugars.