__ CaF2 + __ Na3PO4 = __Ca3(PO4)2 + __NaF
3 CaF2 + 2 Na3PO4 = 1 Ca3(PO4)2 + 6 NaF
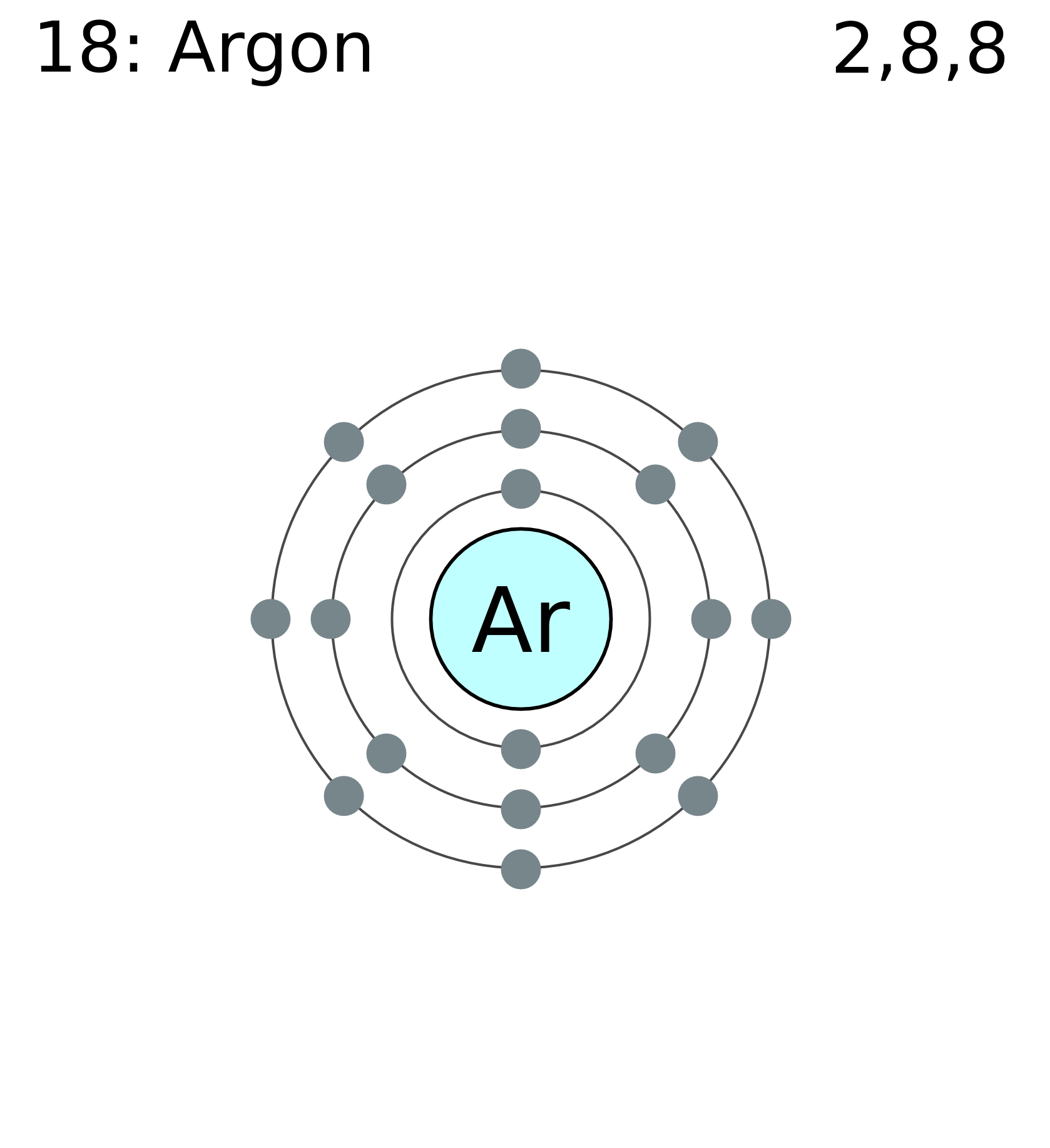
Draw the Bohr Model for Argon.
- 3 shells - 3rd horizontal row
- 18 toatle electrons
- 2 valence electrons in its outer shell - 2nd vertical column
Draw the Lewis Dot structure for Oxygen.

What gas does any alkali metal produce when it is introduced to water?
Hydrogen
How can you determine is a substance is an acid?
If the substance is mixed with cabbage water, if it is an acid, the mixture should turn pink.
__ TiF4 + __ H2O = __ TiO2 + __ HF
1 TiF4 + 2 H2O = 1 TiO2 + 4 HF
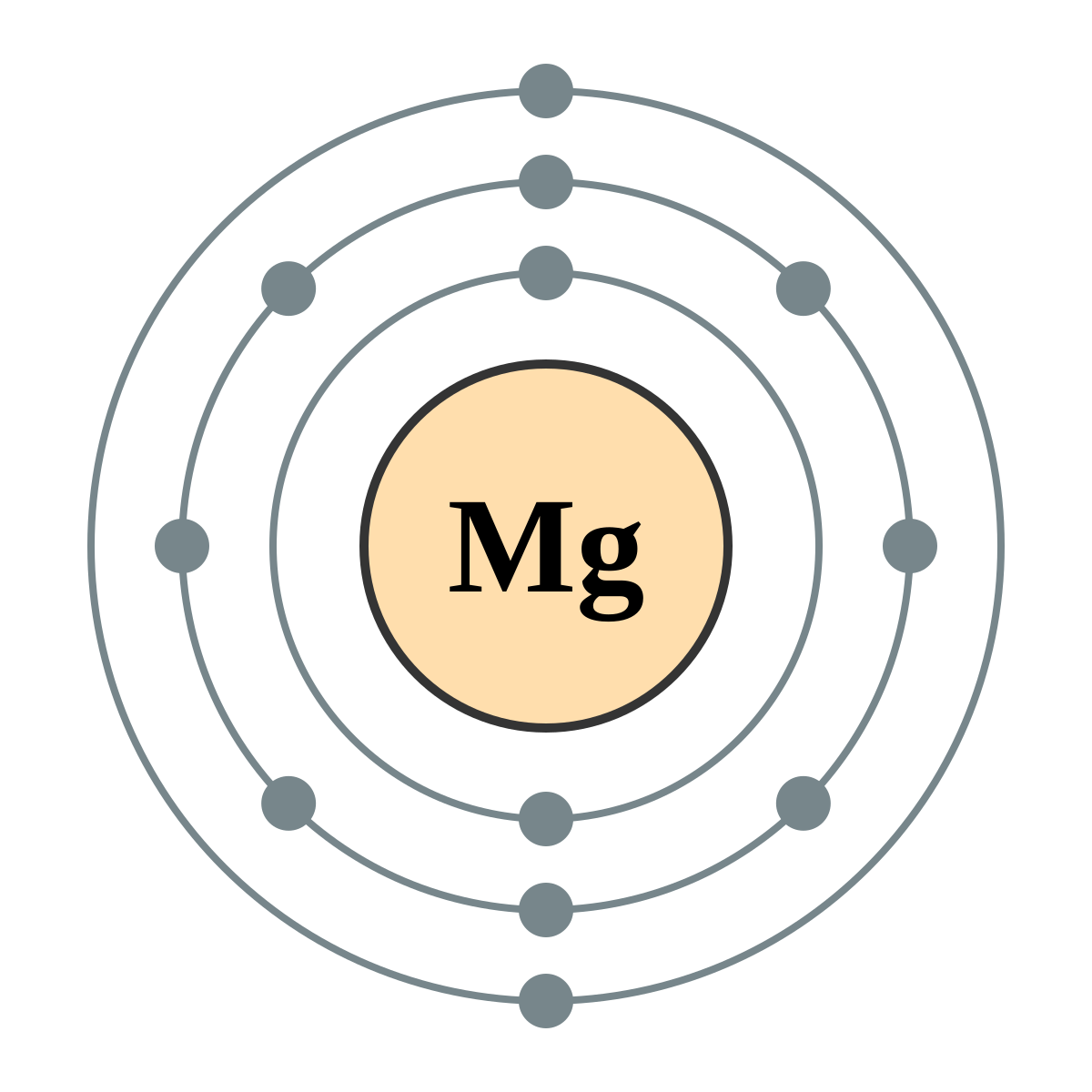
Draw a Bohr Model for Magnesium.
- 3 shells - 3rd horizontal row
- 12 toatle electrons
- 2 valence electrons in its outer shell - 2nd vertical row
Draw a Lewis Dot Structure for Sulfur.

How does any of the alkali metals react to water?
When the alkali metals are introduced to water, the bounce around the water, catch on fire, or explode. All of them produce hydrogen when they are introduced to water.
How can you determine if a substance is a base?
When the substance is mixed with cabbage water, if the mixture turns blue, the substance is a base.
_ Ba3N2 + _ H2O = _ Ba(OH)2 + _ NH3
1 Ba3N2 + 6 H2O = 3 Ba(OH)2 + 2 NH3
Draw a Bohr Model for Potassium.
- 4 shells - 4th horizontal row
- 19 toatle electrons
- 1 valence electron in its outer shell - 1st vertical tall column

Draw the Lewis Dot Structure for Carbon.

What do the alkali metals do when they are cut?
Corrosion occurs and they turn a different color.
Name some substances that are acidic.
(Hint: You can list the ones that were in the experiment / demonstration we did on 9 / 22 / 20)
Lemon Juice
Vinegar
Shampoo
Pink Soap
__ CaF2 + __ Na3PO4 = __ Ca3(PO4)2 + __ NaF
3 CaF2 + 2 Na3PO4 = 1 Ca3(PO4)2 + 6 NaF
Describe how you can determine how many valence electrons are in the outer most shell of any element.
The tall column (vertical column ) the element is on can determine the number of valence electrons any element has in its outer most shell.
Draw the Lewis Dot Structure for Potassium.

Why can't scientist or anyone else introduce Francium with water?
It is to radioactive and dangerous.
Name all of the bases that we listed on 9 / 22 / 20 's Cabbage Water Lab.
Soap
Baking Soda
Salt
Conditioner
Baking Powder
__ Ca + __ O2 = __ CaO
2 Ca + 1 O2 = 2 CaO
Describe how you can determine how many shells any element on the Periodic Table has.
Then horizontal row the element is on determines how many shells the element has.
Draw the Lewis Dot Structure for Lithium and Hydrogen.


Name all of the Alkali Metals.
Hydrogen
Lithium
Sodium
Potassium
Rubidium
Cesium
Francium
List all 9 substances that we used in the Cabbage Water Lab that we did on 9/22/20.
Baking Powder
Baking Soda
Salt
Vinegar
Lemon Juice
Pink Dish Soap
Green Dish Soap
Shampoo
Conditioner