Convert to standard notation: 4.7 X 104
47,000
How many mg are in 47.3 g
47300 mg
What are significant figures used for?
Showing the preciseness of a measurement/ Knowing where to round.
As Chemists we report this measurement as 32.0 because we take measurements from the bottom of the __________.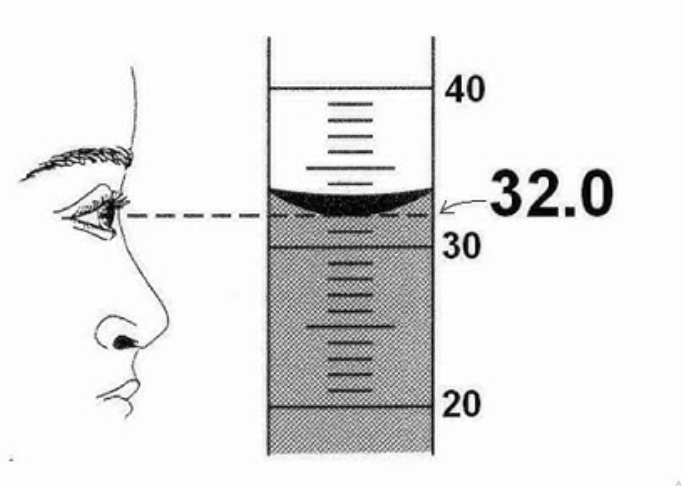
What is the name of this glassware?
And what is the measurement of volume?
And how many sig figs is this measurement?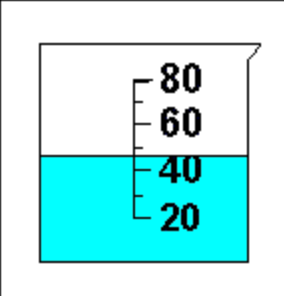
What is beaker
& 47mL (or 46 or 48)
2 sig figs
Convert to standard notation: 9.64 X 10-7
0.000000964
Convert 2500 cm to meters?
25 m ( 1m=100 cm)
How many sig-figs does 1010 have?
What is 3
What does accuracy refer to in laboratory measurements? value.
value.
What is - The closeness to the true value.
What is the volume in this graduated cylinder?
How many sig figs is this?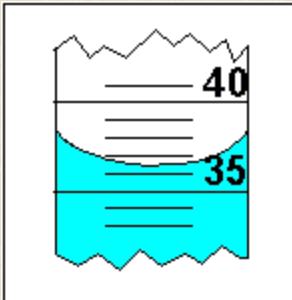
What is 36.5mL. ( an equally precise value would be 36.4 & 36.6)
3 sig figs.
Convert to scientific notation: 0.00094
9.4 X 10-4
How many yards are in 500cm?
5.47yrds
This is the amount of sig figs in this number:
0.00337902270
What is 9.
What term refers to the degree of consistency and reproducibility of measurements?
What is precision
What is the total mass of the object being measured with the triple beam balance?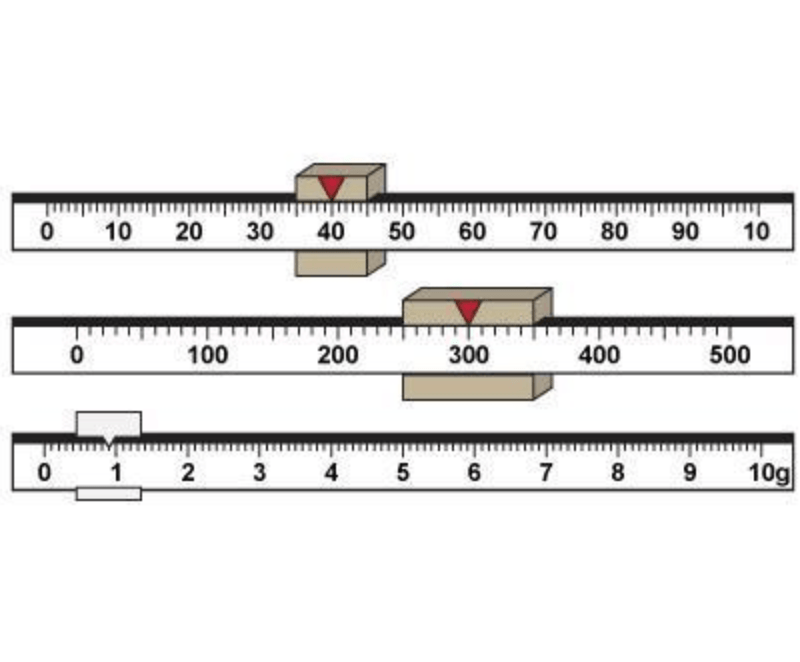
What is 340.9g
A Chem teacher would take points off for this answer:
50×103. Correct it.
What is : should be written as 5.0×104.
Which is heavier 100 kg or 210 lbs?
100 kg
7.90 x 10 46 contains this many sig figs.
What is three.
This is the equation for Percent Error.
What is (experimental - accepted) / accepted * 100
Which measuring apparatus would you use to deliver 9.7 mL of water as accurately as possible? To how many significant figures can you measure that volume of water with the apparatus you selected?
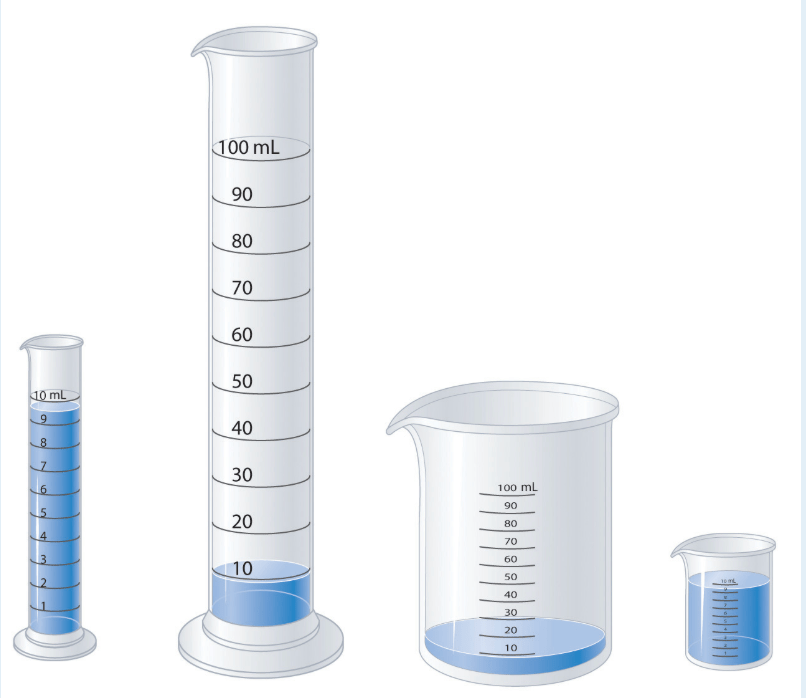
What is the 10mL graduated cylinder.
Which is equal? 6.7 X 103 = 67,000 OR 5.4 X 104 = 54,000
5.4 X 104 = 54,000
Convert 400. miles to inches. (Remember sig figs) AND - put this calculation into scientific notation.
(1 mi = 1609 m)
(1 m = 39.4 in)
What is 25,400,000 inches or
2.54 x 10^7
How many sig figs in this number 0.00048?
What is 2.
Experimental Value = 62.5 g. Accepted Value = 65.2 grams.
What is 4.14% error?
Calculate the mass of an object with a density of 2.78 g/ml and a volume of 48ml.
What is 130 grams.
Convert 50.0 mL to liters.
Conserve sig figs.
Convert to Sci Notation.
What is 5.00 x 10-2
convert 9.7 cm to km
Put the answer in Sci Notation
0.000097 km
or
9.7 x 10-5 km
Report your answer to the correct number of sig-figs.
104.2 + 2.35 =
What is 106.6
Data: 1.11, 1.12, 1.14, 1.15, 1.24, 1.27, 1.33, 1.88, 1.67, 1.45, 1.22
Calculate the % relative range.
What is 58%
What is the volume of the marbles?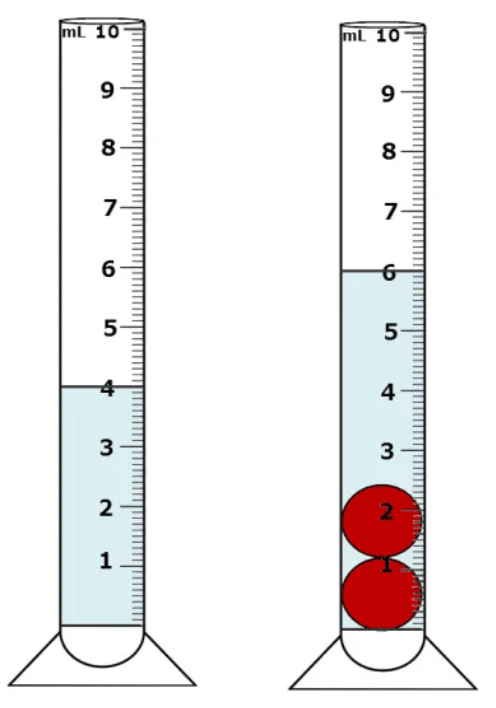
What is 2mL
103 x 10-4 =
And
106 / 10-3 =
What is 10 -1
And What is 109
Convert 6.19 x 105 g to its standard form in kg.
619 kg
Answer the following according to sig figs:
(4.89 - 3.2) x 7.22 =
What is 12.
Data points = 23, 56.7, 25.5, 32, 30, 15 g for mass of a sample.
Follow sig fig rules
Actual = 30g
What is % error?
What is 0%
You measured 6.2mL. Which tool did you utilize for this measurement and why?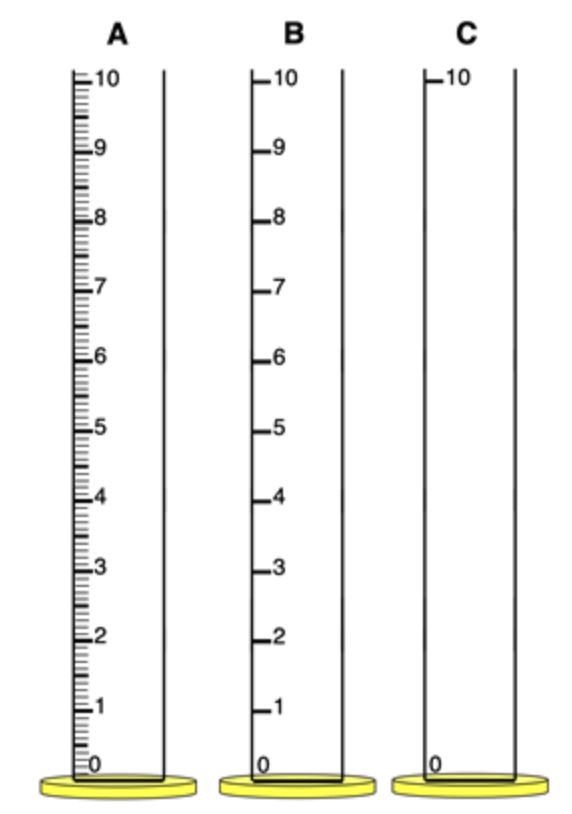
What is B?
The measurement is reported to the tenths decimal place.
The tick marks must have divisions separated by 1 mL. That's cylinder B. With Cylinder B, the volume is known to be between 6 mL and 7 mL. The 0.2 of the reported measurement is the estimated digit.
Report your answer in scientific notation.
5.0g / 10.0 mL=
5.0 x 10-1 g/mL
How many minutes are in a year?
* Can you name the Broadway Musical song that features this answer?*
What is 525,600 minutes in a year
What is Seasons of Love
Report your answer to the correct number of sig-figs.
2.5x100
300
A scientist attempts to calibrate their scale by measuring the mass of a metal cylinder with a known mass of 50.0 g. If the scale’s measurement of the mass is 48.7 g, determine the scale’s percent error.
What is 2.6%
Explain why 43mL and 43.00mL would be incorrect to report for this tool.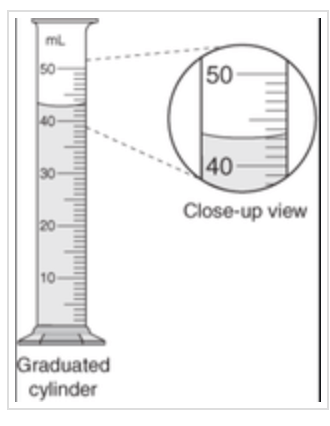
The smallest graduations on this graduated cylinder are to the 1 ml, so the tenth place is estimated; the precision of this graduated cylinder is +/-0.1 ml.
It would be incorrect to report this volume as 43 ml (not enough precision per this instrument) or 43.00 ml (greater precision than this tool allows).