What are the three postulates of collision theory?
1. Atoms/Molecules must collide.
2. Atoms/Molecules must collide with sufficient energy.
3. Atoms/Molecules must collide in the proper orientation
Why would iron filings (powder) rust faster than an iron nail?
They have a larger surface area
Temperature is the average kinetic energy of the particles within a system
If you have 29.8 grams of calcium at 52.0ﹾC that is heated to a temperature of 105ﹾC. How much heat does this require?
1021.9 J
How can a chemist increase the rate of a reaction when the reactants are gases?
Increase the pressure
Use the diagram below to explain which letter choice would have the fastest reaction rate and why using the collision theory.
Diagram C undergoes a successful reaction because the reactants collide with enough energy and in the proper orientation.
How do enzymes in the body speed up reactions?
Enzymes are biological catalysts.
Catalysts lower the required activation energy, making it easier to form the activated complex. This makes the overall reaction faster.
Which is true if ΔH = -10 joules?
A. The system is gaining 10 J and the surroundings are losing 10 J
B. The system is losing 10 J and the surroundings are gaining 10 J
C. Both the system and the surroundings are gaining 10 J
D. Both the system and the surroundings are losing 10 J
B. The system is losing 10 J and the surroundings are gaining 10 J
What is the specific heat of a substance that requires 2531 J to raise the temperature of a 250.0 gram metal rod from 25.0oC to 78.0oC?
0.191 J/goC
What is the metric unit for measuring heat?
Joules
According to collision and transition state theories, what is the importance of activation energy?
It is the energy required for reactants to form the activated complex (or the transition state).
In a lab, students are given 100.0 mL of 1.0 M hydrochloric acid, HCl, to make a product. Which of the following steps should the students take in order to increase the rate of reaction between hydrochloric acid and sodium hydroxide?
Possible answers:
Student could increase the concentration of HCl
Student could increase the temperature of the reaction vessel
Student could add a catalyst to the reaction
What is the potential energy of the activated complex?
450 kJ
A sample of silver with a mass of 70 g is heated to a temperature of 111.3°C and placed in a 151 g container of water at 16.85°C. The final temperature of the silver and water is 19.25°C. Assuming no heat loss, what is the heat transferred from the silver to the water? The specific heat for silver is 0.235J/g°C and water is 4.18 J/g°C.
1514.2 J
How many Joules are in 3.14 cal?
13.1 J
Which reaction would be the MOST difficult reaction to accomplish and why?
Reaction 1, because it takes more energy for the reactants to reach the transition state.
Which number would be effected by the presence of a catalyst in the above reaction?
2
Use the information to answer the following question.
A student is investigating the process of cellular respiration and relates the energy changes involved in the process.
C6H12O6 + 6 O2 -> 6 H2O + 6 CO2 + energy Glucose Oxygen Water Carbon Dioxide
Draw a general potential energy diagram for the above reaction? Explain why you drew it the way you did.
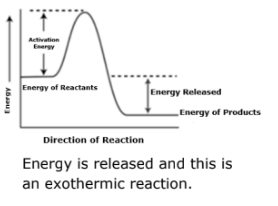
A 75 g of granite is heated and is placed in a calorimeter that contains 95 g of water at 25.0 °C. The final temperature of granite and water is 28.1°C. What is the temperature change of the granite? (cgranite = 0.803 J/goC, cwater= 4.18 J/goC)
- 20.5 OC
How many kJ are equal to 1478cal?
6.178 kJ
Which would dissolve fasts and why?
A would dissolve the fastest.
Small pieces have a larger surface area.
With more surface area, there are more opportunities for collision and more opportunities for a successful reaction.
Use the information to answer the following question.
Several groups of Chemistry students are tasked with producing aluminum chloride using solid aluminum as a reactant. The teacher provides the students with two forms of aluminum, powder and solid chunks.
Which type of aluminum should the students plan to use in order to increase the reaction rate?
Powder because the greater surface area will allow more collisions between reactants to occur.
A + B → C ΔH° = +40 kJ
C + B → D ΔH° = +20 kJ
What would be the final net chemical reaction If these two reactions occur in succession? What would be the overall heat of reaction?
A + 2B → D
60 kJ.
Aluminum 0.897 J/g.oC
Concrete 0.803 J/g.oC
Granite 0.840 J/g.oC
Calcium 0.647 J/g.oC
In the lab, an unknown cube has a mass of 47.0 grams and absorbs 328 joules of energy as its temperature rises from 21.5oC to 30.2oC. What substance from the table could be this unknown?
Concrete
A student is planning to determine the specific heat capacity of iron. To do this experiment the student will need to perform the following procedures:
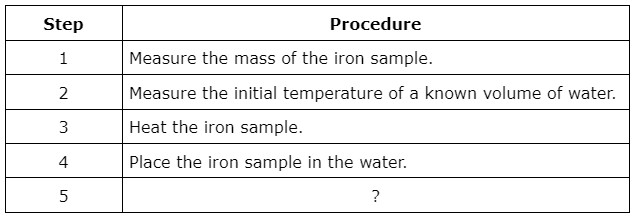
What is Step 5 in the experiment?
Measure the final temperature of the water