Provide the chemical name of ZnCO3
Zinc Carbonate
Metallic bonds can conduct electricity due to ____________
"Sea of Electrons"
Electrons are able to travel easily from one metallic atom to the next
Elements in group 2 has how many valence electrons? What is the group name of these elements?
2 valence electrons, Alkaline Earth Metals
Lewis dot structures only show these types of electrons
valence electrons
intermolecular forces exist between ________ compounds.
covalent
name this compound:
N2O4
dinitrogen tetroxide
How can you tell if a compound is ionic?
If the compound contains a metal and a nonmetal, this is considered as an ionic compound.
Rank the following atoms from smallest to largest in terms of their atomic size:
Li, H, Fr, Cs
H < Li < Cs < Fr
Draw the Lewis structure for water

order the 3 intermolecular forces we learned about from strongest to weakest
hydrogen bonding, dipole-dipole, London Dispersion Forces
If you compare two atoms and you found out that they are "isotopes," what features do they have in common? What features do they differ?
Same number of protons, different number of neutrons
How does an ionic compound conduct electricity?
If the ionic compounds are melted or dissolved, they will conduct electricity due to the presence of ions.
What is the most electronegative element in the periodic table?
Fluorine!!!!
The following diagram shows the transfer of electrons to form what ionic compound? (Please provide the chemical name/formula)
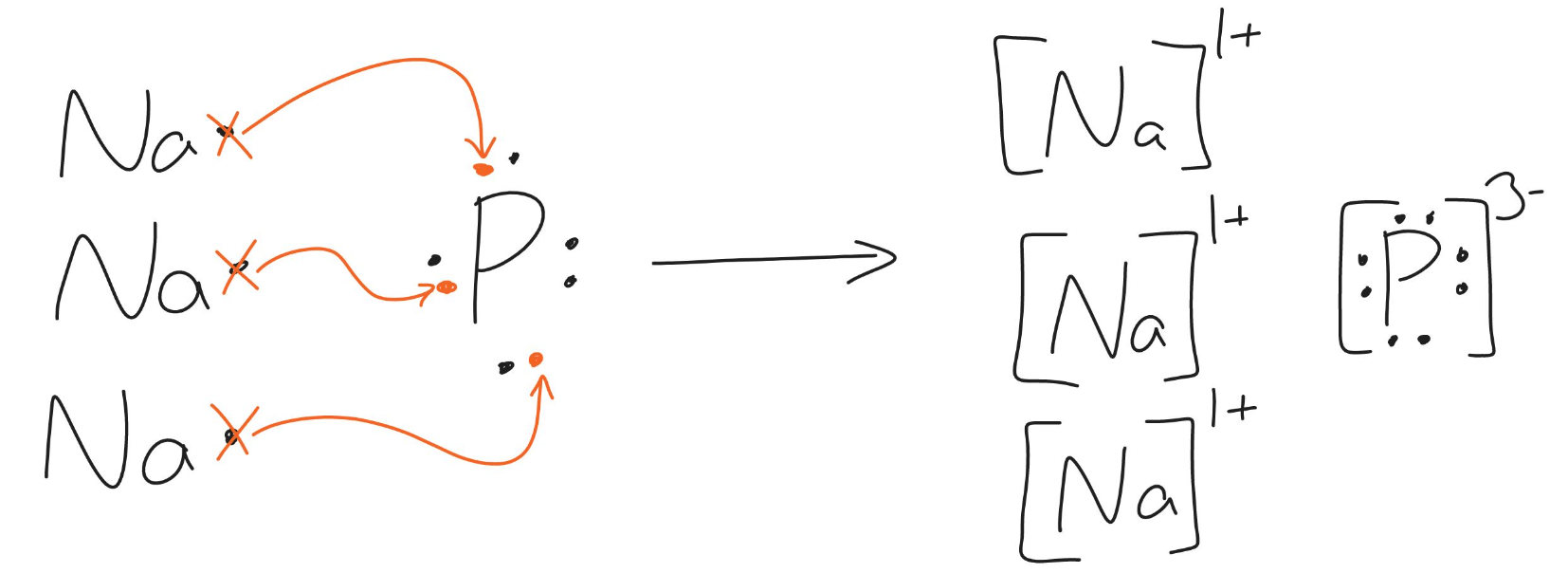
Sodium Phosphide (Na3P)
fluorine, oxygen, and nitrogen are 3 elements that are necessary for the formation of these IMFs
hydrogen bonds
name this compound: Sn(CO3)2
Tin (IV) Carbonate
In order for the sodium metal and fluorine atom to form an ionic compound, which element do they have to attain in order to be stable?
They have to have an electron configuration similar to Neon in order to gain stability (Noble Gas Configuration)
Which of the following elements have similar characteristics with one another? Why?
Ca vs. Ba or S vs. Cl
Draw the lewis dot structure for CaCl2
This is an ionic compound, therefore, you will need to include brackets on the drawing.
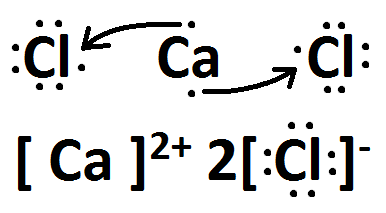
HCl is a strong acid is considered a polar covalent molecule. What is the strongest intermolecular force does this molecule have?
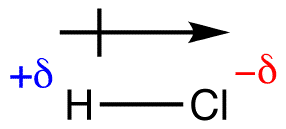
This molecule has dipole-dipole and London dispersion force, however, the strongest force this has is dipole-dipole.
Explain the difference of a molecule and an ionic compound:
Molecule: two or more nonmetallic atoms that share electrons to form a bond
Ionic Compound: two or more atoms (metal/nonmetals) transfers electrons to form a bond
Please provide two examples on what sets an ionic compound apart from a covalent compound.
An ionic compound has a stronger intramolecular attraction as compare to a covalent compound due to the high melting point.
In addition, ionic compounds can conduct electricity due to the presence of ions when melted/dissolved.
Which of the following elements have the same energy level with Boron? Why?
Neon, Aluminum, Helium, or Silicon
Neon, both of the elements are located in the same period (same row)
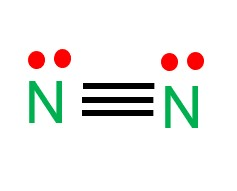
Draw the lewis structure for a nitrogen molecule (N2) and determine its shape
Linear
How do you know if a molecule is considered to be polar or nonpolar?
If the electrons are shared unequally in a molecule then it will be polar.
However, if the electrons are shared equally in a molecule, then it will be considered as nonpolar