A photon has a frequency (n) of 2.68 x 106 Hz. Calculate its energy.
E = 1.78 x 10-27 J
What type of electromagnetic radiation is used by mobile phones?
Microwave
What determines if the electrons are ejected?
The intensity affects the number of electrons, and the frequency affects the kinetic energy of the emitted electrons.
What are the four different orbitals?
s,p,d,f
Write the noble gas configuration for Radium.
[Rn] 7s^2
Calculate the wavelength and energy of light that has a frequency of 1.5 x 1015 Hz
Ans: λ = 2.0 x 10-7 m E = 9.95 x 10-19 J
Which light is refracted the furthest?
Violet
Explain one application of the photoelectric effect in your daily life.
The applications of the photoelectric effect brought us "electric eye" door openers, light meters used in photography, solar panels, and photostatic copying.
 which element does this orbital notation represent?
which element does this orbital notation represent?
Write the noble gas configuration for Bromine
[Ar] 4s^2
A photon of light has a wavelength of 0.050 cm. Calculate its energy.
Ans: E = 3.98 x 10-22 J
Which type of electromagnetic radiation is used to sterilize food?
How does the intensity affect the photoelectric current?
As intensity increases, the photoelectric effect increases
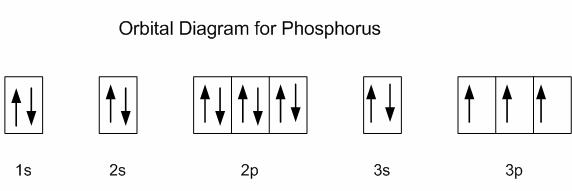
What is the orbital notation for Phosphorus?
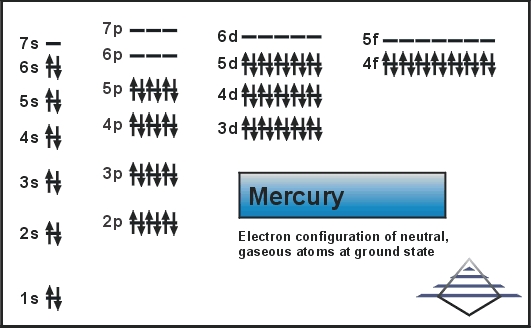
Write a noble gas configuration with an orbital diagram for Mercury
Calculate the total energy in 1.5 x 1013 photons of gamma radiation having l = 3.0 x 10-12 m.
Ans: 1.0 J
Which type of electromagnetic radiation travels faster?
Radio Waves and Gamma waves both travel at the same speed.
What do the negative circles represent in this diagram?
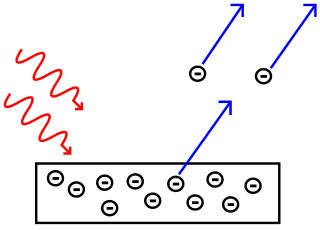
Electrons are ejected from the surface of the metal.
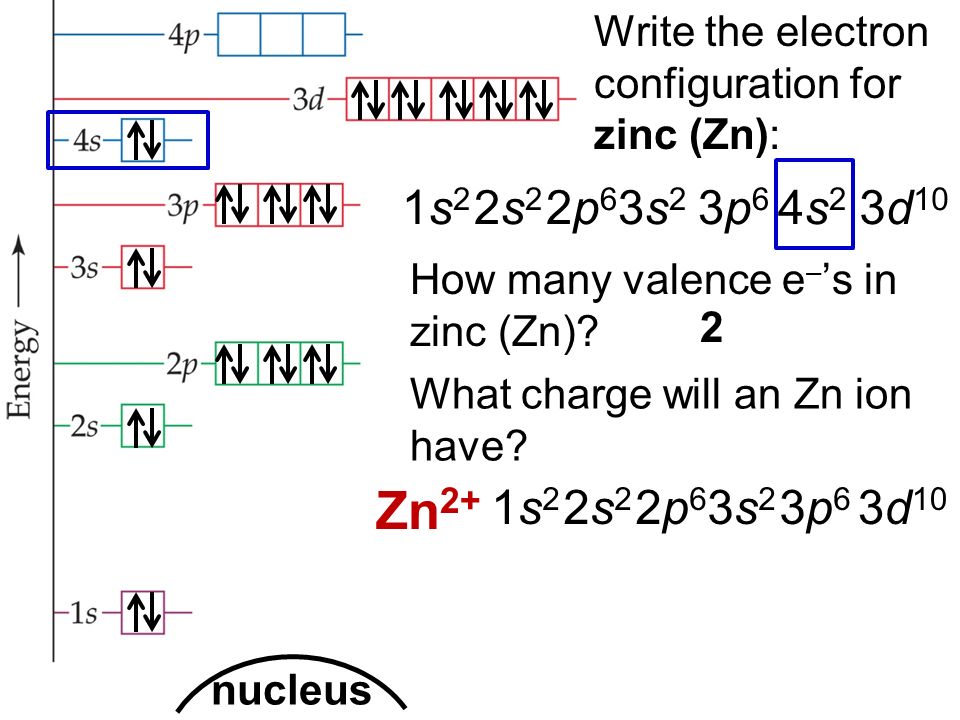
What is the orbital notation for Zinc?
Write complete electron configurations and draw orbital diagrams for strontium.
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2
Calculate the frequency of light that has a wavelength of 4.25 x 10-9m. Identify the type of electromagnetic radiation.
Ans: n = 7.1 x 1016 Hz. UV radiation
Write a sentence that describes the relationship between wavelength and energy of light.
The larger the wavelength the smaller the amount of energy.
Photoelectric effect was successfully explained first by..
Einstein
How is orbital notation different from electron configuration?
The orbital notation gives you more information by showing the electrons as arrows, so you can see the spin of electrons.
Write the noble gas configuration for Tungsten and the orbital notation.
