Which model was the earliest version of the atomic model? (must say last name of scientist + name of model)
Dalton's (Solid) Sphere Model
protons and neutrons
According to this notation, which number represents the mass number?
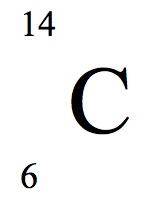
14
The average atomic mass of an element is often different than the mass number. (T/F)
True
How many boxes (orbitals) should a 4d sublevel have?
5
What was the main discovery from the Plum Pudding (Chocolate Chip Cookie) Model?
electrons (negative charge)
Ions are atoms with different number of neutrons. (T/F)
False, electrons
Two isotopes of the same atom have the same number of ______.
protons
What should be done mathematically to the percent abundances of each isotope before calculating the average atomic mass?
Which of the following (a,b, c, d, or e) are/is a correct orbital diagram (following all the rules)?

a and e
Which experiment was responsible for the discovery of the electron?
Cathode Ray Tube Experiment
If an ion has a negative charge, what is changing about the neutral atom?
Cl-21, Cl-30, and Cl-31 all have a different number of ______.
neutrons
Chlorine-35 has an atomic mass of 34.969 amu and chlorine-37 has an atomic mass of 36.966 amu. Chlorine-35 has an abundance of 75.77% while chlorine-37 has an abundance of 24.23%. What is the average atomic mass of chlorine? *must show work to get the points*
35.46 amu
0.7577×34.969=26.50amu
0.2423×36.966=8.957amu
26.50+8.957=35.46amu
Write the noble gas configuration of Sn.
[Kr]5s24d105p2
Ca2+ has (gained/lost) _____ (number) electrons.
lost 2 electrons
How many neutrons does Cu-64 have?
35
What is the missing isotope mass number for an element A if it has an abundance of 9.50%? 4.35% of all the isotopes have a mass of 49.94 amu, 83.79% have a mass of 51.94 amu, and 2.36% have a mass of 53.93 amu. The average atomic mass of all the isotopes is 49.66 amu.
28.42 amu
0.8379 x 51.94 = 43.52
0.0236 x 53.93 = 1.273
0.04350 x 49.94 = 2.170
0.0950 x "X" = ?
43.52 amu+1.273 amu + 2.170 + 0.0950x amu = 49.66 amu
46.96 amu + 0.0950x = 49.66 amu
-46.96 amu -46.96 amu
0.0950x = 2.70 amu x = 28.42 amu
What is the FULL electron configuration of Na+?
1s22s22p6
Explain how the events/results of the cathode ray tube experiment led to the discovery of a particular part of the atom. Be sure to include the events, the results, and the conclusion using reasoning.
The cathode ray tube experiment involved a beam of electricity running through a cathode ray tube. When a positively charged plate was brought near the tube, the beam curved up towards it. When a negatively charged plate was brought towards it, it curved away from the plate. This proved that there was a negative charge in the beam (the electron) that was causing the repulsion between it and the negatively charged plate.
How many total electrons does N3- have?
10 (7e- + 3 e- )
What is the mass number of an isotope of Boron if it has 6 neutrons?
11 (6+5)
A mystery element with 29 protons has two naturally occurring isotopes. One isotope has a mass of 62.93 amu at an abundance of 69.17%. What is the mass of the second isotope?
~66.00 amu
(100%-69.17% = 30.83%)
average atomic mass = 63.55 amu
(62.93 amu x 0.6917) +0.3083x = 63.55 amu
43.53 amu + 0.3038x = 63.55 amu
Which element/ion matches the following electron configuration with an atomic number of 35?
1s22s22p63s23p64s23d104p6
Br-