This element is found in the 3rd period and 14th group.
What is Silicon?
The neutrons and protons are found in this area.
What is nucleus?
This element is found in which group?
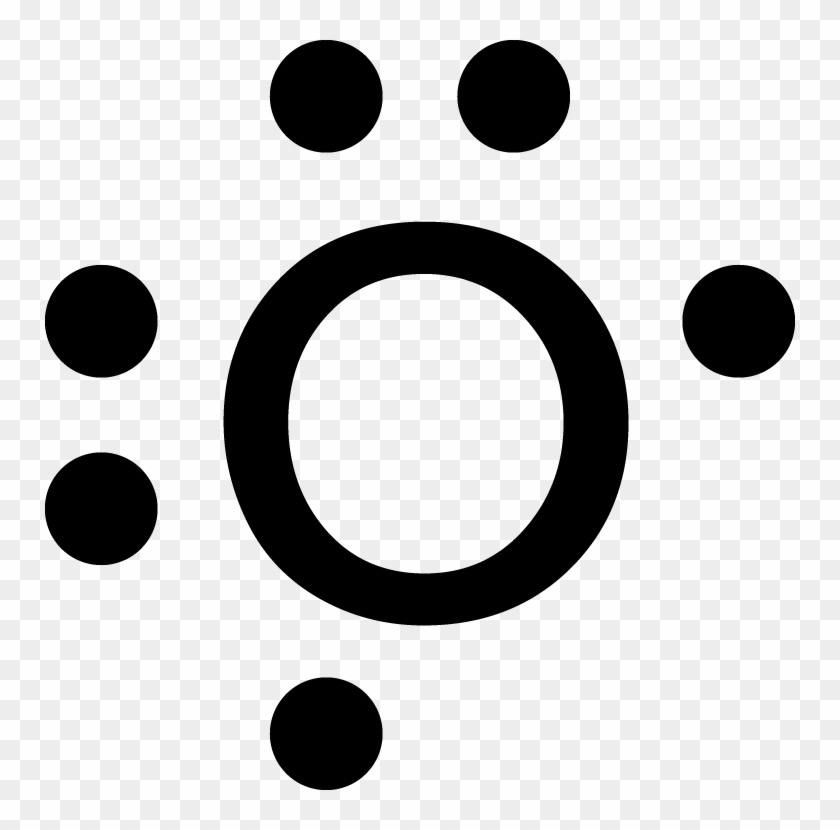
What is 16th?
How many atoms are in SiO2?
What is 3?
This element has 0 neutrons.
What is Hydrogen?
This element has 47 electrons.
What is Silver?
These particles are negatively charged.
What is electrons?
What is this element?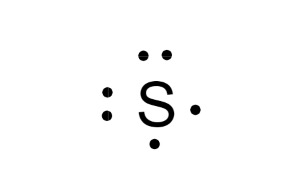
How many atoms of Iron are in FeO3?
What is 1?
The element of Sodium has how many neutrons?
This element has the smallest mass.
What is Hydrogen?
For every period on the periodic table you add.....
What is an orbital?
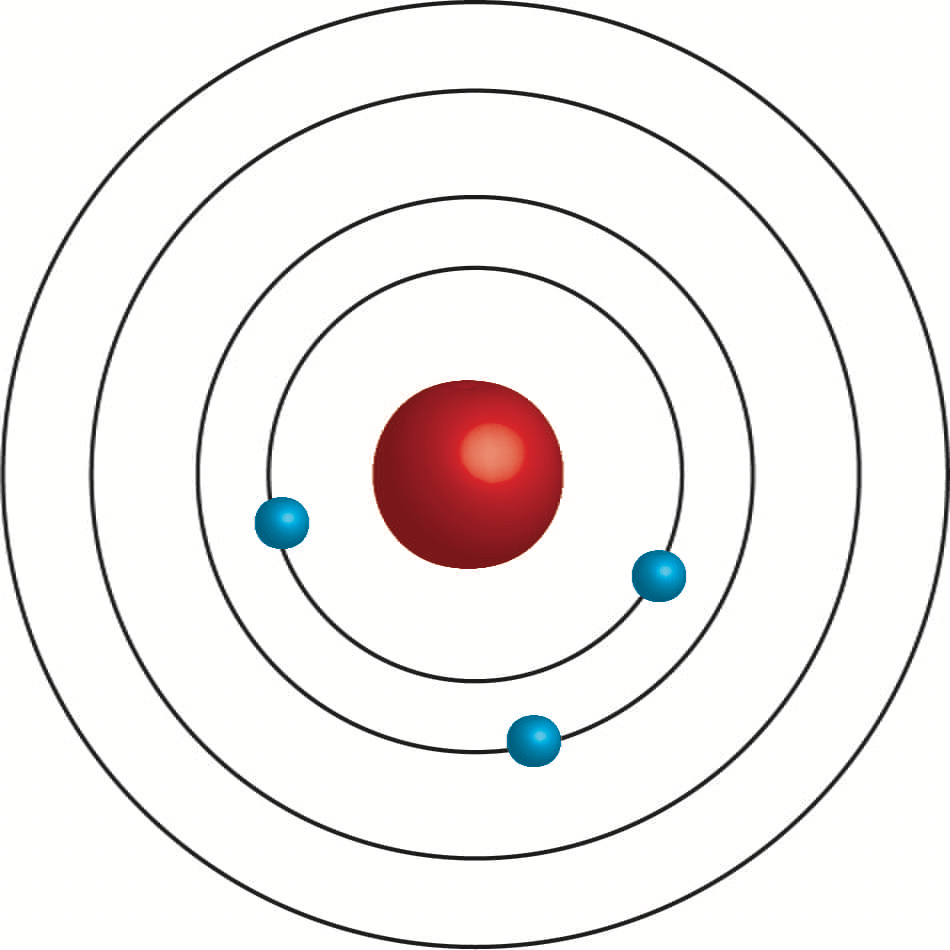
What is Lithium?
How many atoms are in Al2O3?
These two elements both have 6 neutrons.
What is Boron and Carbon?
This element has 22 neutrons.
What is Argon?
This is the sum of two particles in the nucleus.
What is atomic mass?
How many electrons will this element lose or gain?
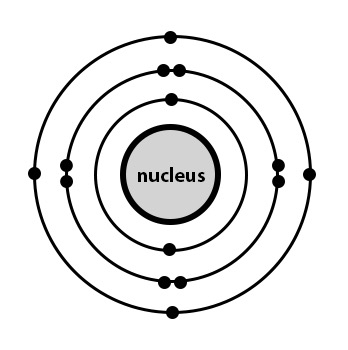
What is Lose 4/Gain 4?
How many atoms are in NaHCO3?
What is 6?
This element has 50 neutrons.
This element is Ms. Geile's favorite.
What is Mercury? (Hg)
These are the outermost particle on an atom.
What is valence electrons?
This element is known as.......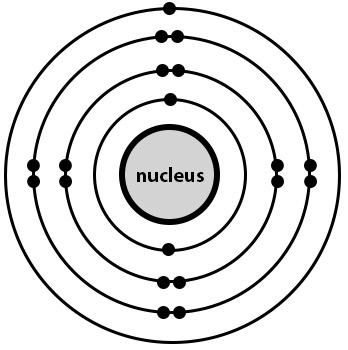
In Be2Al2SiO6, there are how many atoms in total?
What is 11?
How many neutrons are in the element of Fr?
What is 136?