This subatomic particle that determines an element's identity.
What are protons?
The atomic number of this atom: chlorine-35
What is 17?
2 atoms that are isotopes will have _________ and ________ in common but ________________ and _____________ will be different.
What is: 2 atoms that are isotopes will have the number of protons and the number of electrons in common but the number of neutrons and the mass number will be different?
What is jump to a higher energy level?
This color has the shortest wavelength of visible light
What is violet light?
The term given to atoms of the same element that have a different number of neutrons.
What is an isotope?
The name of the element with 20 protons, 23 neutrons, and 18 electrons.
What is Calcium?
The number of protons in the partcle shown below:
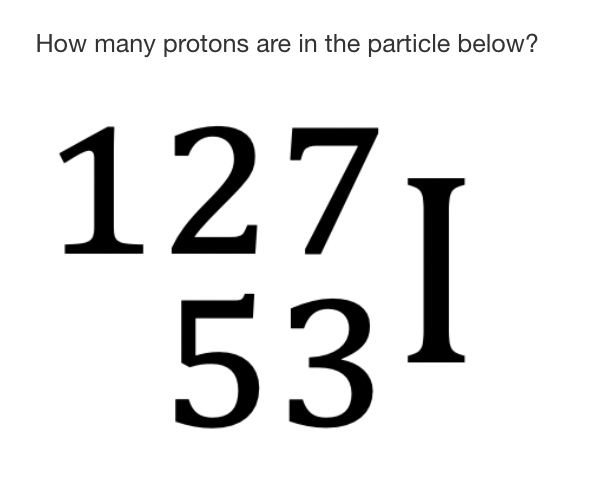
What is 53 protons?
Lithium has two naturally occurring isotopes: lithium-6 and lithium-7.
If the average atomic mass of lithium is 6.941 amu, which isotope is the most abundant?
What is the Lithium-7 isotope?
A ring further from the nucleus has more or less energy than a ring closer to the nucleus.
What is more energy?
Which has a higher energy: green light or orange light?
What is green light?
The term given to atoms of the same element that have a different charge.
What is an ion?
The number of protons, neutrons, and electrons in the following atom
iron-56
What is 26 protons, 30 neutrons, and 26 electrons?
The mass number of an atom of oxygen with 10 neutrons.
What is 18amu?
The calcualated average atomic mass of sulfur if 95.02% of all sulfur atoms have a mass of 32 amu, 0.76% have a mass of 33 amu, and 4.22% have a mass of 34 amu.
What is 32.01 amu?
Sulfur-32: (95.02/100)(32) = 30.41amu
Sulfur-33: (0.76/100)(33)=0.25amu
Sulfur-34: (4.22/100)(34) = 1.35amu
Average: 30.41+0.25+1.35= 32.01amu
The color of light that is produced from a large jump and large fall.
What is violet light?
Light with a _________ wavelength has more energy
What is a shorter wavelength?
This causes an isotope to be radioactive.
The number of protons, neutrons, and electrons in the following atom
magnesium-25
What is 12 protons, 13 neutrons, and 12 electrons?
The TOTAL number of subatomic particles found in the nucleus.

What is 63?
Mass number = protons + neutrons
Mass number = number of subatomic particles in the nucleus
The calculated average atomic mass of Iridium using the information below.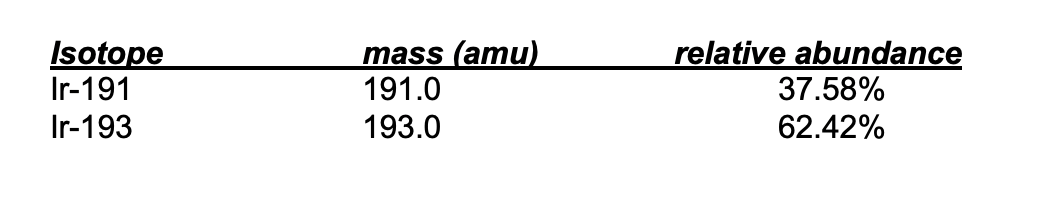
What is an average atomic mass of 192.3 amu?
Ir-191: (37.58/100)(191.0)=71.78amu
Ir-193: (62.42/100)(193.0)=120.5amu
Average Atomic Mass = 71.78+120.5= 192.3amu
The color of visible light that is released when an electron has a small jump and small fall?
What is red light?
An atom release energy in the form of light when this happens.
When an electron moves from a higher energy level (excited state) to a lower energy level?
Atoms on the Periodic Table have this charge.
What is a neutral charge?
List the following information for EACH of the 3 subatomic particles found in an atom:
- The Mass
- The Charge
- The Location
What is the following information:
- Protons - Mass of 1 amu, Positive Charge, Found in the Nucleus
- Neutrons - Mass of 1 amu, Neutral Charge, Found in the Nucleus
- Electrons - Mass of 0 amu, Negative Charge, Found in the Electron Cloud
The number of neutrons shown in the ion below. 
What is 10 neutrons?
Titanium has five common isotopes. This is the calculated average atomic mass of Titanium using the information below.
46Ti (8.3%), 47Ti (7.4%), 48Ti (73.7%), 49Ti (5.4%), 50Ti (5.2%)
What is 47.9 amu?
46Ti: (8.3/100)(46)=3.8amu
47Ti (7.4/100)(47)=3.5amu
48Ti (73.7/100)(48)=35.4amu
49Ti (5.4/100)(49)=2.6amu
50Ti (5.2/100)(50)=2.6amu
Average atomic mass = 3.8+3.5+35.4+2.6+2.6= 47.9amu
The colors of the visible light spectrum, in order, from highest energy to lowest energy.
What is violet, indigo, blue, green, yellow, orange, and red?
The elements that make up the mixture based on the emission spectra shown below
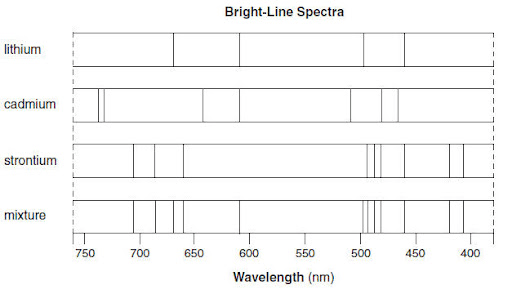
What are Lithium & Strontium?
The smallest particle of a covalent compound is called _________, which is made up of _______________. These elements are located ________________ on the Periodic Table.
The smallest particle of an ionic compound is called _________, which is made up of _______________. These elements are located ________________ on the Periodic Table.
What is...
The smallest particle of a covalent compound is called a molecule, which is made up of only nonmetals. These elements are located to the right of the stairs (along with Hydrogen) on the Periodic Table.
The smallest particle of an ionic compound is called a formula unit, which is made up of metals and nonmetals. These elements are located to the left of the stairs (minus Hydrogen) on the Periodic Table.