Which of the following is the furthest from the center of an atom?
Protons
Neutrons
Electrons
Nucleus
Electrons
Which of the following comparisons correctly describes subatomic particles?
a. an electron has a negative charge and a mass larger than the mass of a proton
b. a neutron has a negative charge and a mass smaller than the mass of a proton
c. a neutron has a neutral charge and a mass larger than the mass of an electron
d. a proton has a positive charge and a mass smaller than the mass of an electron
c. a neutron has a neutral charge and a mass larger than the mass of an electron
An atom has 29 protons, 29 electrons, and 35 neutrons. What is the mass number of the atom?
a. 29
b. 35
c. 64
d. 93
c. 64
The atomic number of an element indicates which of the following?
a. the number of neutrons in the atom
b. the number of protons in the atom
c. the sum of the neutrons and protons in the atom
d. the sum of the protons and electrons in the atom
b. the number of protons in the atom
Which of the following elements is classified as a metal?
a. bromine
b. helium
c. sulfur
d. lithium
d
Which of the following best describes an atom?
a. protons and electrons are grouped together in a random pattern
b. protons and electrons are grouped together in an alternating pattern
c. a core of protons and neutrons surrounded by electrons
c. a core of protons and neutrons surrounded by electrons
Use the picture of an atom below to answer the question.
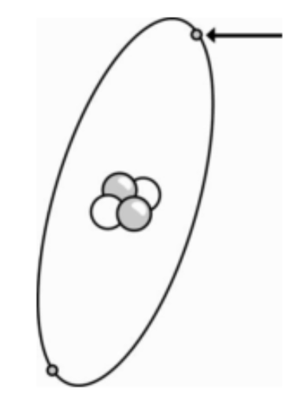
Which statement best describes the part of the atom that is shown by the arrow?
Electron
An atom has 14 protons, 14 electrons, and 15 neutrons. What is the mass number of the atom?
a. 29
b. 35
c. 64
d. 93
a. 29
Based on its position on the periodic table, which of the following elements is a nonmetal?
a. potassium (K)
b. vanadium (V)
c. nickel (Ni)
d. bromine (Br)
d. bromine (Br)
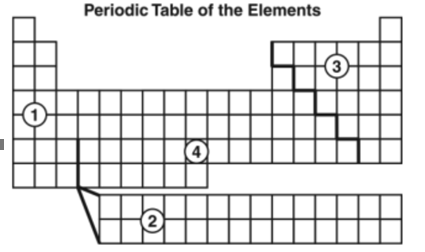
In which region of the table would nonmetals be found?
a. 1
b. 2
c. 3
d. 4
3
Which student correctly identifies the charge of the subatomic particles?
a. Kelly: Protons (neutral), Neutrons (positive), Electrons (negative)
b. Barry: Protons (negative), Neutrons (positive), Electrons (neutral)
c. Protons (positive), Neutrons (neutral), Electrons (negative)
d. Protons (neutral), Neutrons (negative), Electrons (positive)
c. Protons (positive), Neutrons (neutral), Electrons (negative)
Which of the following subatomic particles can be found inside the nucleus of an atom?
a. electrons only
b. neutrons only
c. protons and neutrons
d. protons, neutrons, and electrons
c. protons and neutrons
An atom has 30 protons and a mass number of 65. How many neutrons does it have?
a. 29
b. 35
c. 64
d. 93
b. 35
A
Which of the following statements is true-
a. These elements are in the same period.
b. These elements are in the same group.
c. These elements share the same chemical properties.
d. These elements are both metals.
a
The following statements were made about the parts of an atom.
I. Protons determine the identity of the atom.
II. Neutrons determine the physical properties of an atom.
III. Valence electrons determine the chemical properties of an atom.
a. I only
b. II only
c. I and II
d. I and III
I and III
Which best describes the relationship between subatomic particles in any neutral atom?
a. the number of protons equals the number of electrons
b. the number of protons equals the number of neutrons
c. the number of neutrons equals the number of electrons
d. the number of neutrons is greater than the number of protons
a. the number of protons equals the number of electrons
An atom has 45 protons, 45 electrons, and 48 neutrons. What is the mass number of the atom?
a. 29
b. 35
c. 64
d. 93
d. 93
Group 1 (the alkali metals) includes lithium (Li), sodium (Na), and potassium (K). These elements have similar chemical properties because they have the same ________________.
a. numbers of protons and neutrons
b. numbers of electrons in the outer energy level
c. numbers of protons in the nucleus
d. numbers of neutrons in the nucleus.
B
Which element has a mass number 195 and an atomic number 78?
Platinum
All of the following statements are accurate except-
a. Protons and neutrons have the same mass.
b. Electrons have greater mass than protons.
c. Electrons have less mass than neutrons.
d. Protons and neutrons have a greater mass than electrons.
b. Electrons have greater mass than protons.
Which of the following elements has a proton count of 30?
Zinc
An atom has a mass number of 48 and 24 protons. What is the neutron count?
24 neutrons
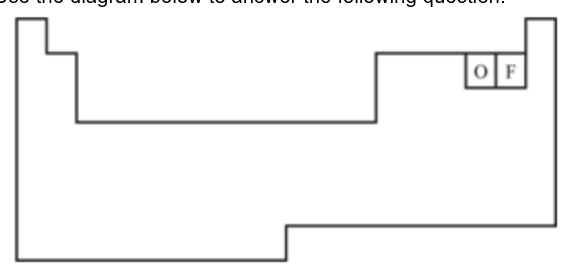
Oxygen has an atomic number of 8. Which of the following elements would you expect to be most similar to oxygen in terms of chemical properties?
a. Nitrogen (N)
b. Fluorine (F)
c. Sulfur (S)
d. Chlorine (Cl)
Sulfur (S)
How many neutrons are in an atom of Bromine with a mass of 80?
45