A liquid which dissolves other substances
BONUS (DOUBLE JEOPARDY): Give an example
What is a solvent?
The two types of chemical reactions
What are anabolic and catabolic reactions?
a) A small molecule
b) A long chain molecule made up of many subunits
a) What is a monomer?
b) What is a polymer?
The four essential macromolecules of life
What are carbohydrates, lipids, proteins, and nucleic acids?
The animal with the fastest metabolism
What is the hummingbird?
The percentage of water in the human body
What is 60%?
The breakdown of glucose is an example of what type of reaction
What is a catabolic reaction?
What molecules are composed of
What are atoms?
The body's primary source of fuel or energy
What are carbohydrates?
a) What element is the MOST common in living things? BONUS (DOUBLE JEOPARDY): Why?
b) What is the 2nd MOST common element in living things?
a) What is oxygen?
b) What is carbon?
The four properties of water that make it essential to life
What are 1) cohesion, 2) adhesion, 3) temperature stabilizing effect, and 4) universal solvent?
The four factors which affect the rate of a chemical reaction
What are 1) concentration, 2) temperature, 3) pH, and 4) enzymes.
True or False: Glucose is a monomer.
What is true?
The type of fat that has a kink (bend) in its molecular structure. These fats are typically liquid at room temperature.
What are unsaturated fats?
a) Type of bonding involving the sharing of electrons
b) Type of bonding involving the transfer of elections
a) What is covalent bonding?
b) What is ionic bonding?
Why water is able to absorb and transfer heat easily.
What is a high specific heat capacity?
The two ways you can denature (unfold) an enzyme.
What are 1) temperature change (high temp.) and 2) pH change?
a) The monomer of carbohydrates
b) The monomer of lipids
c) The monomer of proteins
d) The monomer of nucleic acids
a) What are monosaccharides?
b) What are fatty acids?
c) What are amino acids?
d) What are nucleotides?
The macromolecule that makes up over half of the dry weight of the cell. Antibodies and enzymes are examples of this macromolecule.
BONUS (Double Jeopardy): Name ONE other example of this macromolecule.
What are proteins?
Type of bonding seen in:
a) NaCl
b) CO2
c) H2O
a) What is ionic bonding?
b) What is covalent bonding?
c) What is covalent bonding?
The slogan of NASA's Mars Program
What is "Follow the Water"?
a) The optimal pH for pepsin
b) What would happen if the enzyme, pepsin, was at a pH of 9?
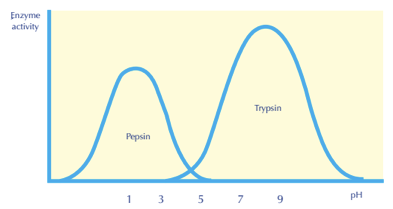
a) What is pH = 1?
b) What is denaturation?
a) The reaction that builds polymers (Hint: it is an anabolic reaction)
b) The reaction that breaks down polymers into monomers (Hint: it is a catabolic reaction)
a) What is dehydration synthesis?
b) What is hydrolysis?
a) TWO negative health effects of eating too many lipids (fats)
b) Name TWO foods that contain lipids
BONUS (Double Jeopardy): What is the scientific name for plaque in the arteries (clogged arteries)?
a) What are heart attacks, strokes, blood clots, coronary heart disease, obesity?
b) What are fried foods, avocados, butter, milk, cheese, fish (Omega 3s), olive oil, vegetable oils, etc.?
The number of naturally occurring elements
What is 92?