Anything that has mass and takes up space.
Matter
What is the formula for density?
Mass divided by volume (D=m/V)
What is the atomic number for Carbon (C)
6
Center of an Atom
Nucleus
What happens to the amount of matter that makes up a human when they are transported to Mars?
Nothing, Stays the same
This form of matter has a definite volume and takes the shape of its container
What is a LIQUID
Given the volume and density of a substance. How do you calculate mass
multiply volume and density
Elements is a column on the periodic table share
Similar properties
List the three subatomic particles and their charges
Proton=positive
Electron=negative
Neutron=neutral (zero)
What physical property is best used to identify an object that contains: Iron, sand, gravel, silica
Magnetism
What are some things you can do to increase the dissolving rate of sugar in water?
Increase temperature, increase speed of stirring
using density in your answer, Why do objects float
Object is less dense than the liquid its floating in
Elements that have properties of both metals and nonmetals are called
Metalloids
How many electrons are in Boron if its atomic number is 5
5
What physical property is best used to identify a substance that is in the liquid state
What happens to the distance and motion of the particles in an object when energy is added to it.
Distance= increases
Motion= increases
Calculate the density of an object with a mass of 45g and volume of 150mL
0.3 g/mL
List the elements is group 2
Be, Mg, Ca, Sr, Ba, Ra
Neon is a gas made up of only one type of atom. Which term best describes neon?
An element
ONE
This state of matter has the highest amount of energy, causing the particle's motion to increase
Gas
Calculate the mass of an object with a density of 3.45g/cc and a volume of 340cc
1173 g
The periodic tables is arranged by the
atomic number
What determines the identify of an atom
number of protons
Effect of temperature on the amount of salt dissolved.
What is the independent and dependent variable?
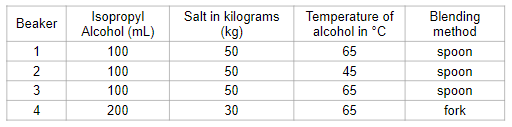
IV: Temperature
DV: amount of salt dissolved