What is the center of an atom called?
Atomic nucleus
What is the property of water that causes water molecules to be attracted to each other?
cohesion
What is the intermolecular force called that forms between water molecules?
Hydrogen bonding
What macromolecule makes up olive oil?
Lipids
What is the positively charged subatomic particle called?
proton
What is the property of water that causes water molecules to be attracted to surfaces of objects (like glass or a penny)?
adhesion
What is the weakest attraction that occurs when electrons zoom past each other on different atoms called?
London Dispersion Forces
Which macromolecule is the main source of energy in your body?
carbohydrates
What subatomic particle in the atom is equal to the number of protons?
electron (number of electrons=number of protons)
Why is the density of ice less than the density of water?
In ice, water molecules are arranged in a crystalline structure. By having this structure, the molecules take up less space than when they are in the liquid state and are able to move more/fill up more space. (The more mass per volume, the higher the density)
Which is the strongest of the intermolecular forces (attractions)?
dipole-ion
What macromolecule is this?
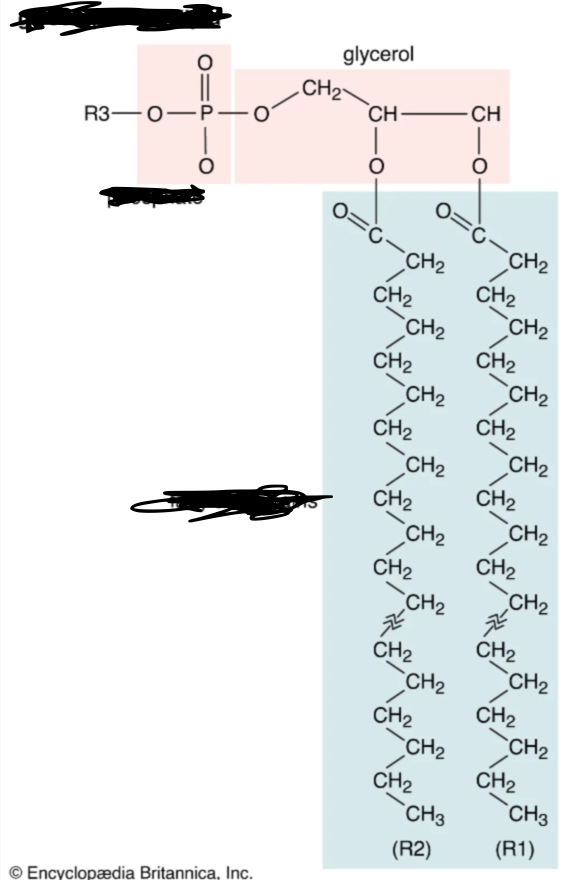
Lipid
What is the atom called when there are more neutrons than electrons?
Isotope
Why does it take longer to heat up water than many other liquids (like alcohol and acetone)?
Water has stronger intermolecular forces between particles than other liquids. It takes more energy (more heat) to break those attractions between particles
Is the hydrogen bond stronger than the covalent bond? Why or why not?
No, hydrogen bond is actually and intermolecular attraction (which is temporary) and does not involve sharing of electrons. A covalent bond is a bond that forms when elements share their electrons with each other
How do you build a carbohydrate macromolecule, starting from elements?
carbon, oxygen, and hydrogen form monosaccharides. Monosaccharides may form disaccharides or polysaccharides. Polysaccharides are the "final" carbohydrates
When an atom loses or gains an electron and becomes charged, what is it called?
ion (like Na+ or Cl-)
What causes the polarity of water?
Polarity of water is caused by its dipole moment (an uneven distribution of between the negative oxygen and positive hydrogens)
If bonds are stronger than intermolecular forces, why do ionic compounds (which have ionic bonds) dissolve in water (which have hydrogen bonds)?
The total amount of dipole-ion attraction between the water molecules and the ions in the ionic compound, overcome the ionic bond
What are the three types of lipids found in our bodies?
Triglycerides, cholesterol, and phospholipids