This is the term to describe the sum of protons and neutrons in an atom.
What is mass number?
This is the term to describe an atom with a positive charge due to the loss of an electron.
What are cations?
This is the term to describe the attraction of molecules of the same substance.
What is cohesion?
This is a macromolecule that contains a glycerol molecule and three fatty acid chains.
What is a lipid?
This is the type of chemical bond found in a molecule of NaCl (sodium a metal and chlorine a nonmetal).
What is an ionic bond?
This is the term to describe the number of protons and electrons in a neutral atom of an element.
What is atomic number?
This is the term to describe a chemical bond between two nonmetals.
What is a covalent bond?
This is the term to describe the attraction of molecules of different substances.
What is adhesion?
This is a monomer used to build proteins.
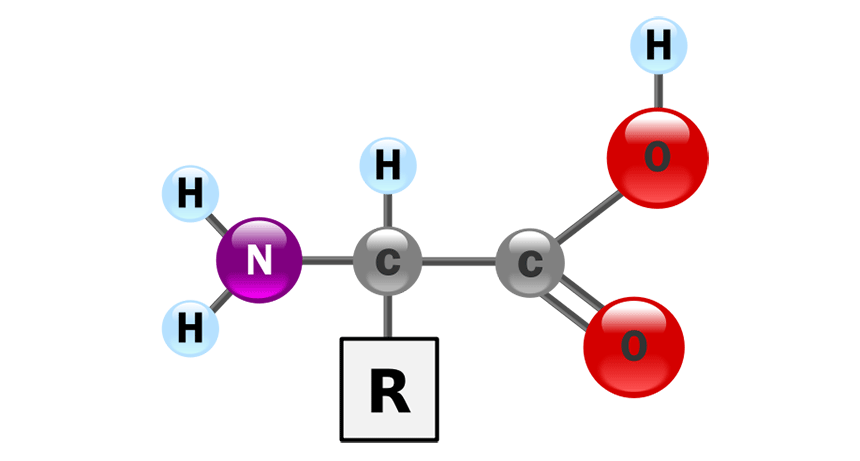
What is an amino acid?
This is the macromolecule that is primarily found in fats and oils.
What is a lipid?
These are positively-charged particles located in the nucleus of an atom.
What are protons?
This is the term to describe the chemical bond between a metal and a nonmetal.
What is an ionic bond?
This the term to describe a liquid solution that has a higher concentration of H+ ions than OH- ions.
What is an acid?
This is a macromolecule that serves as the main source of energy in the body.
What are carbohydrates?
This is a liquid solution that has a higher concentration of OH- ions than H+ ions. This liquid solution has a pH greater than 7.
What is a base?
These are negatively-charged particles located on the outside of the nucleus in an atom.
What are electrons?
This is the term to describe two atoms of the same element that have a different number of neutrons.
What are isotopes?
This is the term to describe a property of water that explains why more energy is required to heat up water compared to a metal.
What is heat capacity?
This macromolecule is found in this structure.

What are nucleic acids?
This is a type of covalent bond that results when electrons are shared equally. The molecule looks even.
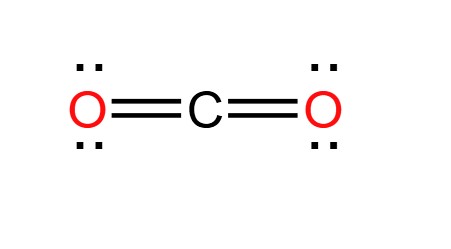
What is a nonpolar molecule?
What are nonmetals?
This is the type of chemical bond found in a molecule of carbon dioxide (CO2).
What is a covalent bond?
This is a property of water that explains how objects such as insects or paper clips can float on a thin film (instead of sink). The cohesive nature of water supports this.
What is surface tension?
This macromolecule forms enzymes such as lactase.
What are proteins?
This is a property of water that refers to water being the liquid that substances are frequently dissolved in.