Where are the metals on the periodic table?
The left of the staircase
Which of these describes the amount of energy required to remove an electron from an atom?
A)Atomic Radius
B)Electronegativity
C)Ionization Energy
D)Reactivity
C) Ionization Energy
The label for a chemical was lost. A chemist conducts a flame test to figure out the identity of this compound. The flame test resulted in a purple flame, what compound is it based on the data?
Strontium Chloride - Red
Copper Sulfate - Green
Potassium Nitrate - Purple
Sodium Chloride - Orange
Potassium Nitrate
100 points EACH
What equipment would be the best for measuring the mass of a rock in grams?
What would be best for measuring how many milliliters of liquid you have?
What will be best for measuring how many centimeters long a necklace is?
Electronic Balance
Graduated Cylinder
Ruler
Elements in the same ___________ will have the same properties
Group (up and down!)
What is the definition of electronegativity?
Ability to attract electrons in a chemical bond
BONUS HISTORY (DOUBLE POINTS):
What two observations from the gold foil experiment lead to the development of the NUCLEAR model of the atom?
1) Atom is mostly empty space
2) Atom has a positively charged center (nucleus)
What discovery resulted in the plum pudding model being developed?
The discovery of the electron
(JJ Thompson was able to remove a small negatively charged particle from the atom)
Which group is the most stable and why?
Noble gases, they have a full valence shell (8) and are unreactive
Which element has the highest ionization energy?
a) I
b) Br
c) Cl
d) F
What is the CHEMICAL SYMBOL for this electron configuration:
1s2 2s2 2p6 3s2 3p1
Al
Which two atoms/ions are the same element?
Atom A: 5p, 6n, 5e
Atom B: 6p, 6n, 5e
Atom C: 5p, 5n, 6e
Atoms A and C because same protons
protons give an element its identity
What group is element 1s2 2s2 2p6 in?
Noble gases
What is the trend for atomic radius across a period and why does it occur?
Atomic radius DECREASES across a period because number of protons increases, so force of attraction pulling the electrons is stronger (pulls them closer so the atom is smaller!)
An electron is excited to a higher energy level with 2.09 volts of energy. When it returns to the ground state, how much energy will be released as a photon of light?
2.09 volts
How much water is there in the graduated cylinder below?
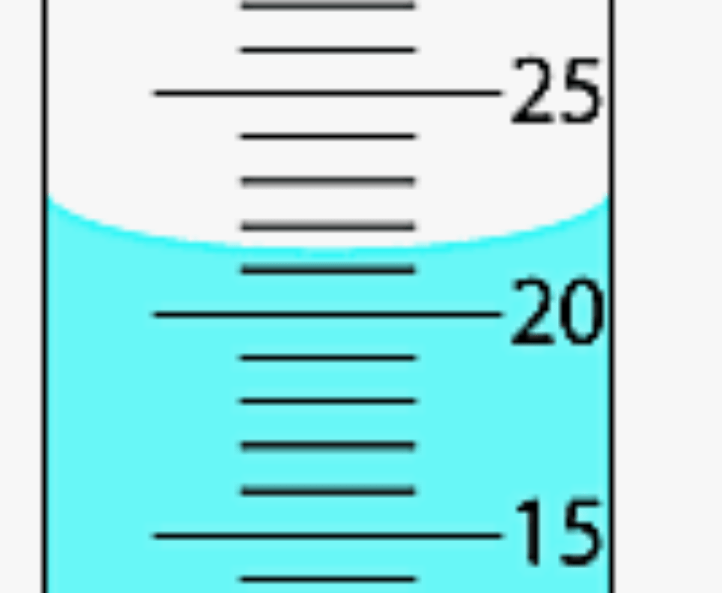
21.5 mL (or 21.6 mL, 21.4 mL)
What is the group name that has elements with the oxidation number of +1?
Alkali Metals
What element will have the same properties of Sodium and will also have a larger radius?
Potassium, Rubidium, Cesium, or Francium
What's the electron configuration for Arsenic?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
Sodium has an average atomic mass of 22.99 amu according to the periodic table.
1) What is the most abundant isotope of sodium?
2) How many neutrons will a sodium atom most likely have?
1) Sodium - 23
2) 12 neutrons
Which sample is the metal, metalloid, and nonmetal?
Sample A: dull, brittle, poor conductor
Sample B: lustrous, brittle, can conduct electricity
Sample C: lustrous, malleable, can conduct electricity
A: Nonmetal
B: Metalloid
C: Metal
Draw two arrows showing the directions in which atomic radius INCREASES on the periodic table.
One arrow going down, one arrow pointing to the left
All elements in group 7 have ____ (number) valence electrons and are _______________ (metals, nonmetals, or metalloids). The group name is _______________.
7, nonmetals, halogens
What is the average atomic mass for an element that has an isotope with a mass of 107 and a percent abundance of 51.86% and 109 with an abundance of 48.14%? What element is this?
107.87 amu, Silver