Multiple Choice:
The pressure exerted by a gas in a container depends on:
the space between the molecules
the number of collisions between gas molecules and the walls of the container
the instrument used to measure the pressure
the number of collisions between gas molecules and other gas molecules
2) the number of collisions between gas molecules and the walls of the container
What is condensation?
Create a particle drawing of a solid, liquid, and gas and label them. Make sure to accurately show the movement and spacing.

How do gas particles exert pressure?
Gas particles are constantly moving around and bumping into things. When they collide with the walls of a container, they push on them.
What is a force?
A Push or Pull
Explain why the alcohol level in a thermometer rises when it is placed in a warmer fluid.
As the warmer fluid particles bump and collide with the glass of the thermometer, energy is eventually transferred into the liquid inside the thermometer. When the liquid gains heat energy, the particles move faster and further apart, known as thermal expansion. Since the thermometer is a closed system, the only way the liquid can expand is up or down.
Create a particle drawing that shows the difference between how particles in a food coloring dye move in hot vs. cold water.

If you have gas particles inside a container, what would happen to the pressure exerted by the gas if the container's volume was decreased?
The pressure inside the container would increase because it would cause the particles to collide more with the container.
The word describing how high or low you are in the sky is referred to as the
Altitude
Explain why the alcohol level in a thermometer drops
when it is placed in a cooler fluid.
As the warmer fluid particles inside the thermometer bump and collide with the glass of the thermometer, energy is eventually transferred from the liquid to the surrounding fluid. As energy is transferred from the thermometer to the fluid, the alcohol particles in the thermometer lose energy and move slower and get closer together known as thermal contraction. Since the thermometer is a closed system, the only way the liquid can move is up or down.
Which state of matter has the most space between particles?
a) solids
b) liquids
c) gases
d) they all have the same space
c) gases
Create a particle drawing showing why a marshmallow gets bigger and smaller inside a syringe when you pull it back and forth.
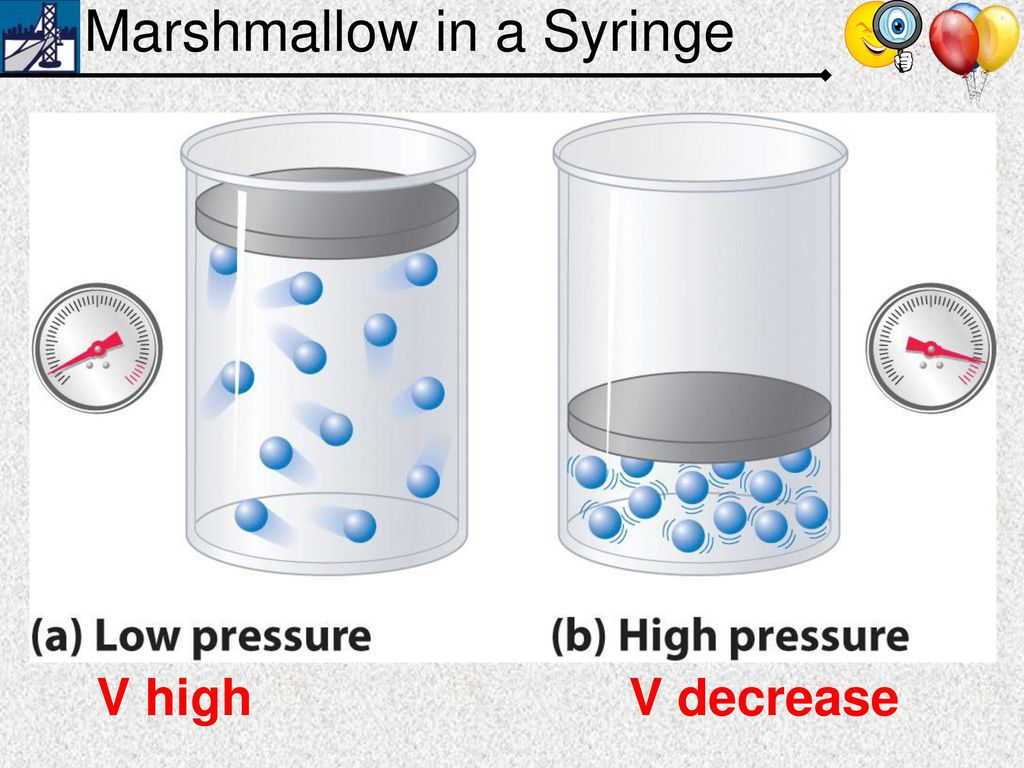
What are 3 assumptions about how gases behave according to Kinetic Molecular Theory?
Gases behave like tiny spherical objects in constant, random motion
These particles move in a straight line until they collide with another particle or the walls of the container
These particles are much smaller than the distance between particles. Most of the volume of a gas is therefore empty space.
4) There is no force of attraction between gas particles or between the particles and the walls of the container. This means gas particles do not “stick” to other particles due to how fast they are moving.
5) Collisions between gas particles or collisions with the walls of the container are perfectly elastic. None of the energy of a gas particle is lost when it collides with another particle or with the walls of the container.
6) The pressure of a gas is related to the frequency and impact of the collisions of the gas particles with the sides of the container in which they are enclosed.
The pressure exerted by the weight of all of the gases in our air is called
atmospheric pressure
Temperature is a measure of energy (hot fast the particles are moving).Heat is the transfer of energy from hotter particles to colder particles
When we did the lab, we put both hands in the same temperature water bath (room temp, 20 degrees Celsius). Even though the water bath was the same temperature,we felt hot in one hand and cold in the other because of heat was entering our colder hand, and leaving our hotter hand.Hotter particles move faster than colder particles and transfer energy to them when they collide.
Which state(s) of matter take on the shape of their container?
Liquids and Gases
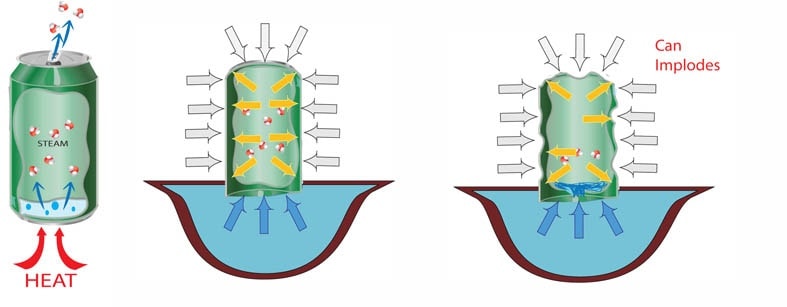
Create a particle drawing explaining why a soda can crushes when water is poured inside, heated, and then flipped into cold water.
Interpret this graph. Write a claim explaining the relationship between altitude and air pressure.
As altitude increases, air pressure decreases.
The process of adding heat to a solid substance to change it into its gas form is called
a) melting
b) condensation
c) evaporation
d) sublimation
d) sublimation
In order for a particle to stop exerting pressure, what must the temperature be in:
Celsius - ?
Kelvin - ?
Celsius: -273.15 C
Kelvin: 0 K (aka ABSOLUTE ZERO!)
Create a model or diagram showing how heat is related to changes in the state of matter using the following words:
- Melting/Freezing
- Condensation/Evaporation
- Sublimation/Deposition
- Adding Heat / Removing Heat
Create a particle drawing explaining how and why the tanker car crushed. Be sure to include a few phrases that describe what is happening in your picture.
Must include:
-How cooling steam from the steam cleaning caused changes in pressure on the inside
- How gas particles exert pressure inside and outside

11) A line of best fit generated from a temperature and pressure graph has the following values:
Y = MX + B
M = 0.371
B = 101.35
What is the temperature when there is 0 pressure based on this line?
Y = 0.371X + 101.35
0 = 0.371X + 101.35
-101.35 = 0.371X
Divide both 0.371X = -101.35
X = -273.18
This word describes the movement of particles from an area of higher concentration to an area of lower concentration. It happens because particles are constantly moving and colliding with each other, causing them to expand into lower concentrated areas with more space. An example of this would be an air freshener or perfume being sprayed through the air.