What is the main point of discussion in the kinetic molecular theory?
Particles (how particles behave/travel in a certain space)
How do cold particles move compared to warmer particles? Why?
Cold particles move slower because they have less energy. Warmer particles move faster because they have more energy
Which is the independent variable on this graph?
Variable 1
How many significant figures?
12000
2 (trailing zeros are not signficant)
Complete the sentence:
An object (particle) that is in motion....
stays in motion until acted on by another force
Why did the ethanol rise higher up the tubing than water did?
Ethanol has a lower specific heat and it is less dense than water. Therefore, the particles needed to absorb less energy than water to move
What kind of correlation is shown on the graph?
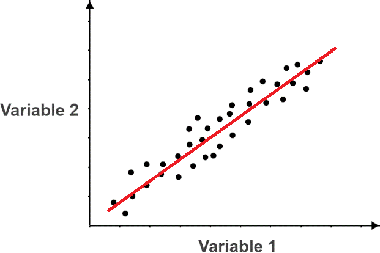
Positive correlation (as independent variable increases, it causes the dependent to increase)
How many significant figures?
14000.
5 (when there is a decimal at the end, all digits are significant)
what is pressure? (explain in terms of particles)
force created by the amount of collision between particles and the walls of a container per unit area, and then between particles themselves
What is this called and how does it work?

Barometer. As atmospheric pressure increases and pushes on the mercury, the mercury rises up the tube.
What graphs have this correlation, from unit 2?
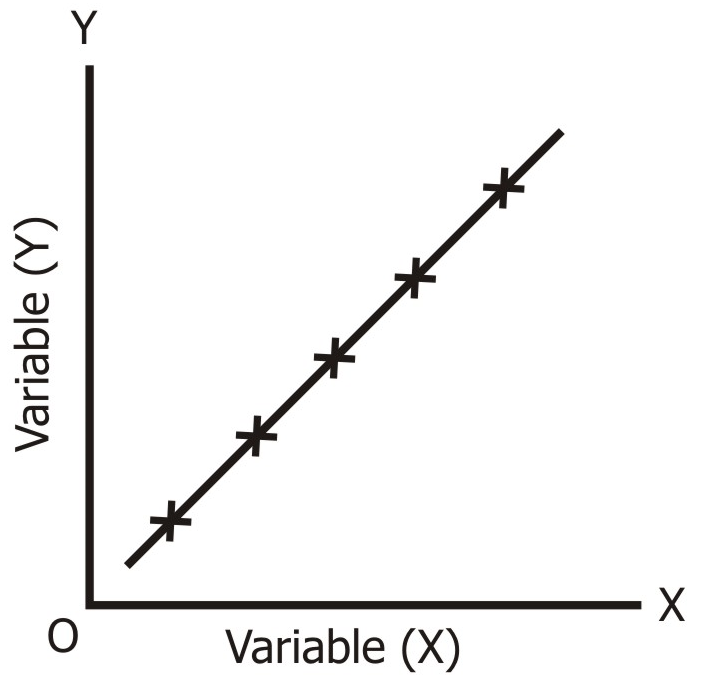
Pressure vs. Temp
Pressure vs. Particles
Volume vs. Temp (thermal expansion)
How many sig figs should there be in final answer?
120 + 100=
1 sig fig! (200)
Why do heavier gas particles move slower than lighter particles?
Heavier particles require more energy to move than lighter particles
What are 3 examples of exothermic reactions from labs 5-7?
Lab 5: your hand releasing heat in cold water
Lab 6: hot can and steam releasing heat in cold water
Lab 7: fire burning is exothermic
(particles did absorb heat which is endothermic, but there was more release of heat than absorbing of heat in total)
What graph is this, of the ones we learned in unit 2? Explain the correlation
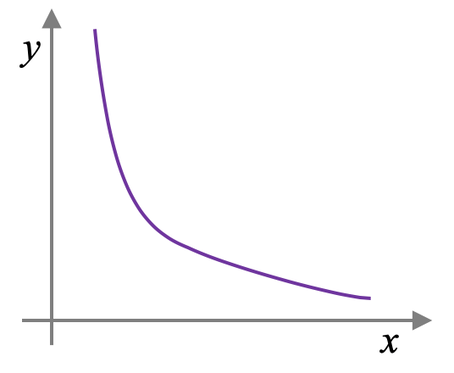
Pressure vs. Volume (as volume increases, pressure decreases)
How many significant figures should there be in final answer?
12x2.0 =
2 sig figs! (24)
(Multiplication! 2 is the least amount of sig figs. Round to least amount)
Why do heavy and light particles produce the same amount of pressure, even though heavier ones travel slower than the lighter ones?
Pressure is a force. Force produced by the particles is the same due to this formula:
Force= mass x acceleration
What are 3 examples of endothermic reactions from Labs 5-7?
Lab 5: your cold hand in hot water (absorbed heat)
Lab 5: ethanol and water absorbing heat from hot plate
Lab 6: can absorbing heat from hot plate
Explain what this graph is showing
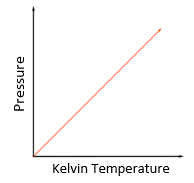
Absolute temperature is defined as the temperature at which there is zero (in reality- minimal) pressure. So at 0 kelvin temperature, the pressure is 0. As kelvin temperature rises, so does the pressure
What is the answer (with correct sig figs)?
6.5 x 2.00 +5.100
18