Are phase changes and dissolving chemical or physical changes?
Physical
What is the density of a solid with a volume of 200 cm3 and a mass of 180 g?
0.90 g/cm3
Phase with a definite shape and volume
Phase with a definite volume but an indefinite shape
Phase without a indefinite shape and volume
Phase without a indefinite shape and volume with electrical charges
Solid
Liquid
Gas
Plasma
What happens to the temperature during a phase change?
Remains the same
Define chemical and physical properties
Physical- can be seen using the 5 senses
Chemical- must undergo some kind of chemical change, reaction, or describes the chemical make up and cannot be witnessed using the 5 senses
Which is an indication that a chemical change is taking place?
A. Iron changes color when heated.
B. Gas bubbles form in boiling water.
C. Solid wax forms when melted wax falls in ice water.
D. A gas forms when vinegar and baking soda are mixed.
D. A gas forms when vinegar and baking soda are mixed.
You have Two samples of gold with a density of 2.27g/cm3. If you cut the gold in half, what will the density be of both halves?
2.27g/cm3
What does the kinetic theory of matter State?
The more thermal energy a system has, the faster the particles will move and the further apart they will be. The less thermal energy a system has, the slower the particles will move, and the closer they will be.
Which statement best describes the motion of water molecules as the water changes state from steam to liquid?
A. The molecules move faster and occupy more space.
B. The molecules move slower and occupy more space.
C. The molecules move faster and occupy less space.
D. The molecules move slower and occupy less space.
D. The molecules move slower and occupy less space.
Which fact about water is an example of a chemical property?
A. Water freezes when it expands.
B. The boiling point of water is 100°C.
C. Water can separate into hydrogen and oxygen
D. The density of water is greater than the density of ice.
C. Water can separate into hydrogen and oxygen
Students performed an investigation on how aluminum cans taken to a recycle center were able to be reused. They found out an aluminum can undergoes which type of change and why?
a physical change because the material remains the same
An object was put into a graduated cylinder holding a volume of water equal to 300 mL and the height of the water rose to 350 mL. The mass of the object was 1500 g. Solve for volume then solve for the density of the object?
30 g/mL
Which statement about a ring of pure gold is not consistent with the particle theory of matter
A. The particles are all identical.
B. The particles have spaces between them.
C. The particles are not in motion since it is a solid.
D. The particles are held together by electric forces.
C. The particles are not in motion since it is a solid.
As you increase in energy, what are the phase changes on the diagram if you start as a solid?
melt and boil
Which of the following are physical properties? (Can be more than one)
solubility, luster, malleability, combustibility
solubility, luster, malleability
Josie investigated chemical and physical changes using eggs. She did the following four actions to four different eggs.
- Boiled
- Cracked
- Fried
- Scrambled
Which action describes a physical change?
Cracked
While washing some berries, a student observes that the blueberries sink in water and the strawberries float in water. What conclusion can you make?
A blueberry has greater density than a strawberry.
An ice cube melts on a hot summer day because the energy of the particles...
A. decreases over a period of time and is lost.
B. increases and the particles move apart.
C. causes them to lock together.
D. is converted into potential energy.
B. increases and the particles move apart.
What is the phase change when an object goes from a solid to a gas, skipping the liquid phase?
Sublimation
Label the pH scale (Acid, Base, and Neutral)
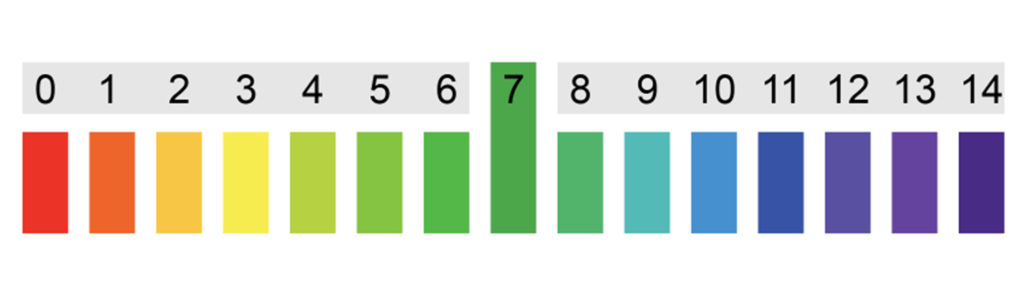
0-6.9 Acid
7 Neutral
7.1-14 Basic
Which one shows one example of a physical change and one chemical change?
A. freezing water and burning coal
B. rusting of iron and baking a cake
C. dissolving powder and shredding paper
D. rusting of iron and baking a cake
A. freezing water and burning coal
A student investigated the density of various liquids. He used water, vegetable oil, and liquid soap in the investigation. He put 2 liquids in each beaker. What can you conclude from the image?

Vegetable oil has the lowest density and liquid soap has the highest density.
Put the States of matter in order based on decreasing energy (highest energy to lowest)
Gas, Liquid, Solid
Endothermic reactions (release, absorb) thermal energy
Exothermic reactions (release, absorbed) thermal energy
Endothermic reactions absorb thermal energy
Exothermic reactions release thermal energy
The table below shows the physical properties of selected metals.
A cube of an unknown metal has a volume of 2.25 cm³ and a mass of 16.2 g.
Based on data in the table below, what is the identity of this metal?

Chromium