What is negative?
The atomic number is equal to the number of these
Atomic radius will do this when you go down a group.
What is increase?
Identify the two atoms that are isotopes of each other
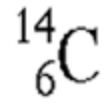


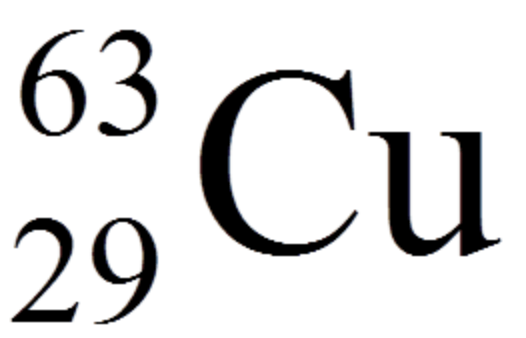
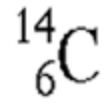

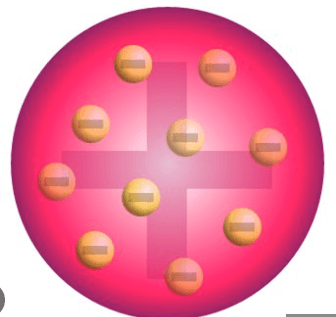
This scientist concluded that the atom looks like plum pudding
JJ Thomson
Protons have this type of charge
These two subatomic particles make up the mass number
What are protons and neutrons?
This is the reason why atomic radius decreases as you go left to right across a period.
The number of energy levels stays constant, but the increasing positive charge in the nucleus draws in the outermost energy level more.
Isotopes have a different number of these
Neutrons
This scientist concluded that the electrons travel in orbits and exist in different energy levels or shells
Niels Bohr
Neutrons have this type of charge
The number of electrons in Aluminum in its neutral state
What is 13?
This is how a cation forms
What is removing electrons?
Write out the formula for average atomic mass
((%1)(mass1) + (%2)(mass2) +...+(%n)(massn))/100
OR
(f1)(m1) + (f2)(m2) + ... + (fn)(mn)
Explain the experiment that was used to help Rutherford make his conclusion
Gold foil experiment: Shot positive alpha particles through a sheet of gold atoms -- some bounced back, some straight through, some deflected. Demonstrated that atoms have a dense and positively charged nucleus
These two subatomic particles are located in the nucleus
What are protons and neutrons?
Oxygen has approximately this many neutrons.
What are 8 neutrons?
Out of the 2 elements below, this element has a higher electronegativity.
1. Iodine (I)
2. Chlorine (Cl)
What is Chlorine (Cl)?
You wouldn't be accounting for the abundances of each isotope
What is the correct order of atomic models?
Electrons are located in this part of the atom
What is orbitals/energy levels/around the nucleus?
What is iron?
Calculate Average Atomic Mass for Boron
10B: 20%, 10amu
11B: 80%, 11amu
Average atomic mass = (%1)(mass1) + (%2)(mass2)
(0.2)(10) + (0.8)(11) = 10.8 amu
Which experiment was used to help a scientist discover that there are negatively charged particles in an atom? Explain this experiment
Cathode Ray tube: ray shot through a tube, the ray of particles was attracted to a positive magnet, showing that negative particles exist and are spread out through atoms
This is the number of protons in this element:
What is 10?
This is the group name for group 1A.
What are alkali metals?
This is the definition of ionization energy
What is the energy required to remove an electron from an atom?
The average atomic mass of Ag is 107.88 amu. It has 2 isotopes, Ag-106 and Ag-107. Ag-107 has an abundance of 51.839%. Calculate the abundance of Ag-106
48.16%
On your whiteboard, draw Thomson's atomic model.