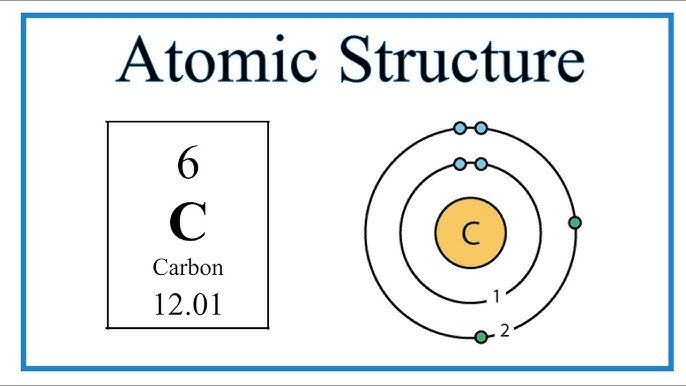
What is an atom?
The smallest unit of matter.
What is an ion?
A charged atom. An atoms that's gained or lost electrons.
What is an isotope?
Atoms with the same number of protons but different number of neutrons.
What are the 4 orbitals and how many electrons do they hold?
s = 2
p = 6
d = 10
f = 14
Given 1s2, what does the number 1, letter s and superscript 2, each tell us.
Number designates the level the electrons will go on. Letters s, p, d or f designate the orbitals and the superscript tell how many electrons are present at that orbital.
Where can you find the atomic number? Is it the larger or smaller number.
Periodic table, it is the smaller of the two numbers.
What are the two types of ions? What are their charges?
Cation-positive and anion-negative.
There are two ways to notate an isotopes. Show both notations for carbon-13.
C-13 or 13C
Which of the following represent the same element.
a. 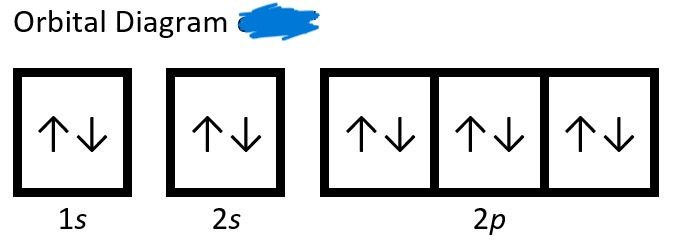
b.
c. [Ne]
d. 1s2 2s2 2p6 3s1
a & d, both represent 11 e- (Sodium).
Based on the picture below, identify the element and the number of valence electrons it contains.
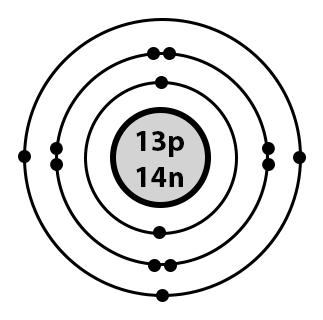
Aluminum; 3 valence electrons
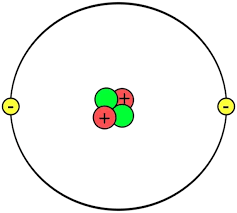 Given the following image, what element is this?
Given the following image, what element is this?
Helium
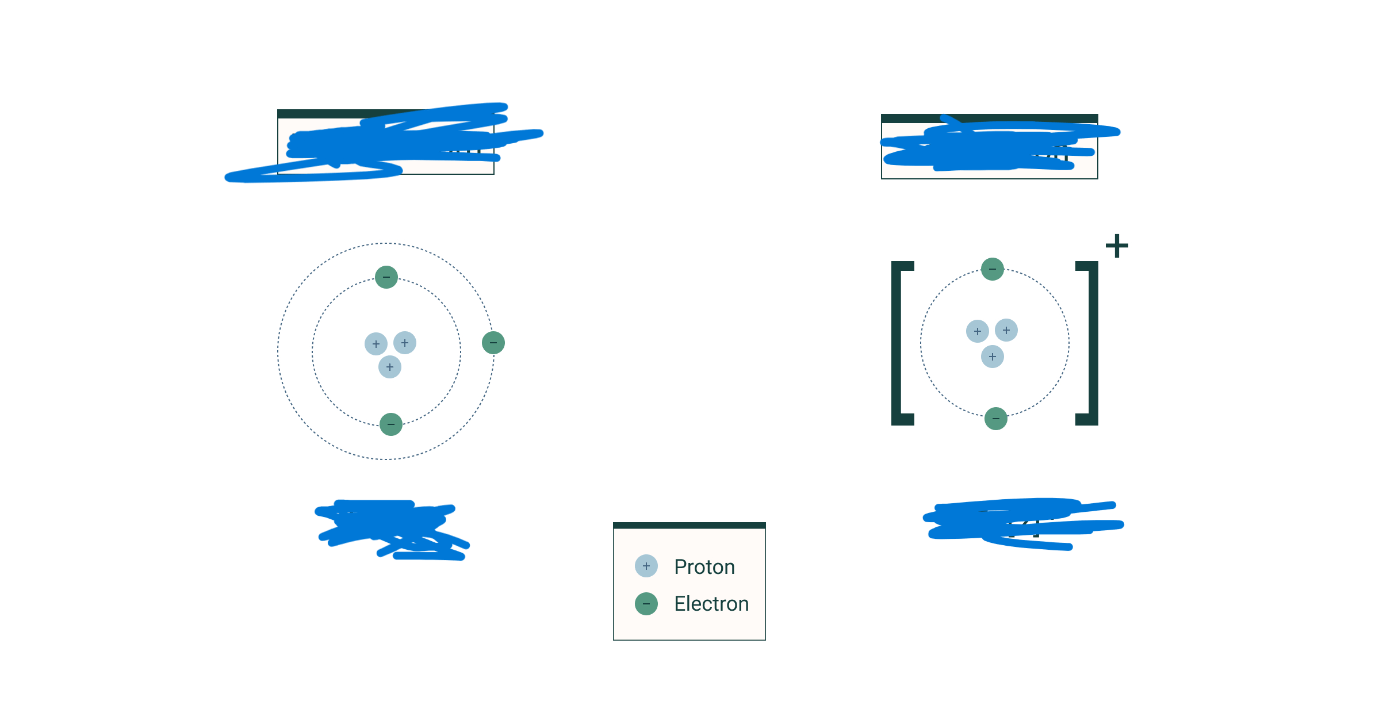
Given this image, write element name and correct symbol.
Lithium. Li+1
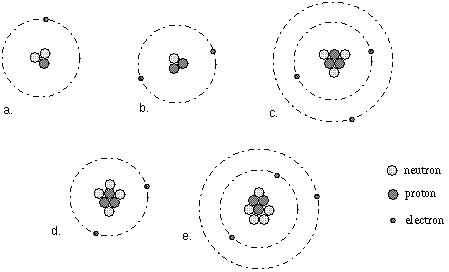
Which of the following are isotopes of one another and why?
C, D and E are isotopes because all of them have the same number of protons (3). They are all isotopes of Lithium.
Which of the follow orbital diagrams is correct?
For the incorrect orbital diagram, explain why this is wrong.

Example 1 is incorrect. Electron must fill each "seat" before pairing. This is because they repel one another.
Draw the Bohr model for Magnesium.

What is the charge of an atom? What has to be true to make this happen?
Atoms have zero charge (neutral). Protons must equal electrons.
An element has 15 protons and 18 electron, is this an ion? If so, what type? Correct symbol?
Ion. Anion. P-3
 Which of the following are isotopes and why?
Which of the following are isotopes and why?
B and C are isotopes. They are Lithium isotopes. They have the same protons but different neutrons.
Bonus: Which of the following are ions?
Write the orbital diagram and electron configuration for oxygen.
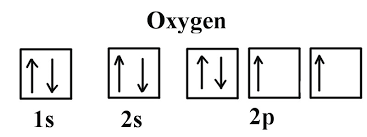
1s2 2s2 2p4
What is wrong with bohr model? Draw the correct model. 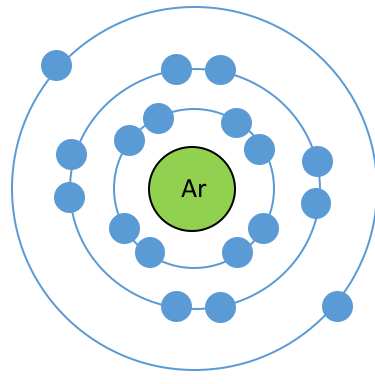
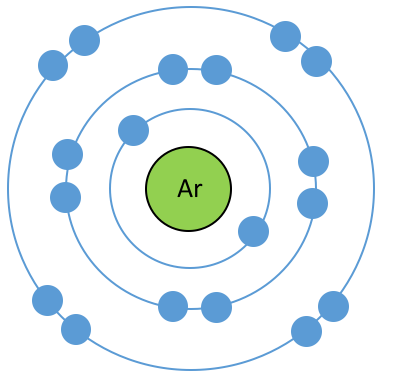
What is the difference between the atomic mass and mass number? Where are they located?
Atomic mass is the average mass of the isotopes found on the periodic table. This is the mass of an atom. Mass number is the mass of the specific isotope. It is not found on the table but given.
Ex: X-m format
e= 28 p=29
p=29 e=27
Given the information above, are these the same element? If so, what element? What are their correction symbols/notation?
Yes; they are both copper. Cu2+ and Cu+1.
How many neutrons does the isotope Neon-22 have?
Show your work on the board
Neon-22
22 (mass) - 10 (atomic #) = 12 neutrons
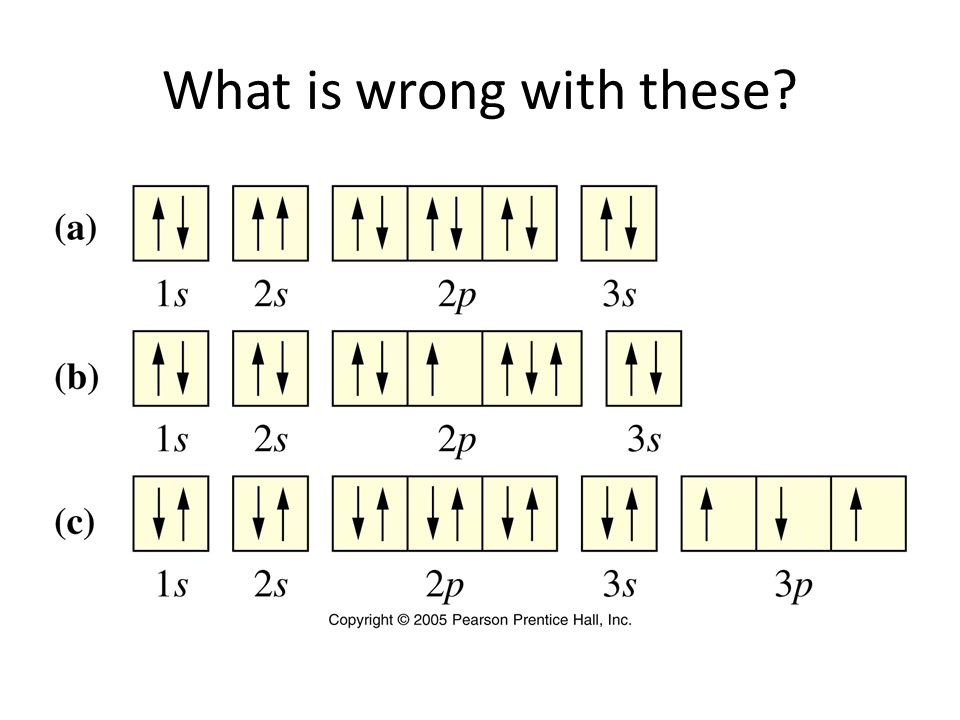 Based on the picture, decided what is wrong with each one.
Based on the picture, decided what is wrong with each one.
A: 2s orbital arrows should be one up and one down
B: 2p orbital has 3 arrows in one orbital; orbitals only carry 2 electrons
C: 3p orbital arrows should all be facing up
A chemistry student draws the Bohr model for Carbon. The class knows carbon has 6 electrons, when neutral. The student places 4 electrons on energy level 1 and 2 electrons on energy level two. Is their Bohr model draw correctly? Why or why not? Draw it out on the board
Energy level 1 can only carry 2 electrons and energy level 2 can carry up to 8 electrons.