What is chemistry?
Chemistry is the study of matter.
Name one scientist involved in making the atomic theory and list on thing they did.
Dalton- atomic theory
Thompson- plum theory
Rutherford- Nucleus
Bohr- Orbitals
What is an ionic compound?
It is a compound with a metal and non-metal and they transfer electrons.

What is a chemical reaction? How do we know one has occurred?
Mixing of chemicals and the creation of something new.
Colour change
formation of gas

Why do we want to control the speed of reaction?
If we are making something we want it to be faster.
What is a physical property? Give an example
A property that can be measured or observed. example: Melting temperature, luster, colour

What to subatomic particles are in the nucleus?
Proton and Neutrons

What is a covalent compound?
It is a compound that has 2 non-metals and they share electrons.

What is Conservation of mass?
Mass can not be created or destroyed, if you start with 10g you end with 10g.
What are the two major types of mixtures?
The two major types of mixtures are heterogeneous and homogeneous mixtures.
What is a chemical Property? Give a example.
Describes how something reacts with something else. Combustibility

What subatomic particle travels around the nucleus.
Electrons

What is the name for CaCl2?
Calcium Chloride
What are the reactants in the following combustion reaction?
C3H8(g)+5O2(g)→3CO2(g)+4H2O(g)
C3H8(g)+5O2(g)
What is one way to speed of a reaction?
- Surface area of reactants (ie. Powder form more than solid mass)
- Concentration of a reactant or adding more reactants (this means there less water and more concentration of chemicals in the solutionà think about making a powdered energy drink- a higher concentration means more powder, so sweeter)
- Higher temperature
- Stirring/mechanical mixing and movement
- Catalysts- chemicals added into a reaction that only speed up the reaction, don’t participate in the reaction
What is a family on a the periodic table?
Vertical group that has similar chemical properties.
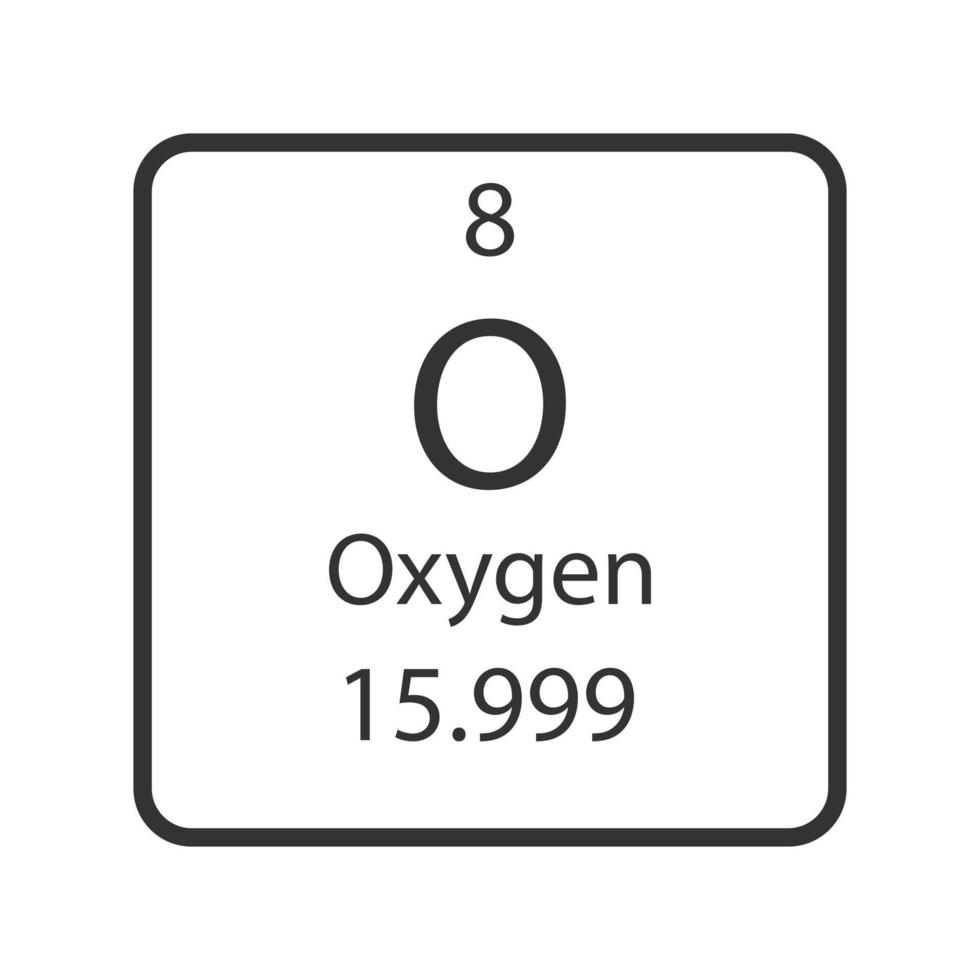
How many protons does oxygen have?
atomic number 8 = 8 protons
What is the formula of trinitrogen hexasulfide?
N3S6
How many carbon and hydrogen are in the following formula?
C3H8
Carbon= 3
Hydrogen= 8
What is the difference between a pure substance and a mixture?
A pure substance is any substance that cannot be broken down further by physical means (such as elements and compounds)
A mixture is any substance that can be physically separated and or broken down (their constituents are not combined chemically).
What is the definition of matter.
Matter is defined as anything that takes up space and has a mass.
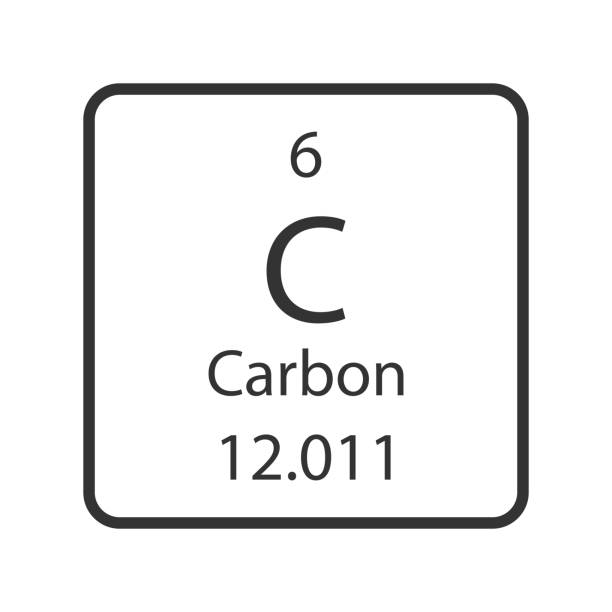
How many neutrons does carbon have?
12-6=6
What is the name of S2O2?
disulfur dioxide
What type of reaction is the following?
C(s) + 2H2(g) → CH4(g)
Formation Reaction
What is one way to slow down a reaction?
- Surface area of reactants (ie. Powder form more than solid mass)
- Concentration of a reactant or adding more reactants (this means there less water and more concentration of chemicals in the solutionà think about making a powdered energy drink- a higher concentration means more powder, so sweeter)
- Higher temperature
- Stirring/mechanical mixing and movement
- Catalysts- chemicals added into a reaction that only speed up the reaction, don’t participate in the reaction