A liquid is a phase of matter that has a _________ volume, and a _______________ shape.
(un)defined
A liquid is a phase of matter that has a defined volume, and a undefined shape.
True or False:All physical changes are reversible
False: Ex- cutting your hair is irreversible.

Is the above a heating or cooling curve?
What is a cooling curve?
This is a state of matter in which the material has a definite volume, but not a definite shape (fits the shape of the container)
What is a liquid?
Phase change from solid to gas
What is sublimation?
This physical property is defined as how much matter is in a specific amount of space.
What is density?
Water boiling into steam is this type of change (explain)
What is a physical change? (Phase changes do not change the chemical composition of a material)
In which segments are phase changes occurring?
What are 2 and 4?
What is the temperature (in Celsius) at which water begins the vaporization/condensation process?
100 degrees C
Water vapor changing to frost on a windshield is an example of
What is deposition?
A solid is a phase of matter that has a _________ volume, and a _______________ shape.
(un)defined
A solid is a phase of matter that has a defined volume, and a defined shape.
Iron rusting is this type of change
What is chemical (a new substance is formed)?
This is the phase change occurring at 4

What is vaporization?
This phase of matter retains its shape and volume and has the strongest attractive forces
What is a solid?
If an object has a mass of 3.67 g and a volume of 8.2 mL, what is its density? (Units and SFs required)
0.45 g/mL
List two chemical properties of matter (Explain each)
What is flammability, reactivity, combustibility...etc.
Combining vinegar and baking soda is this type of change
What is a chemical change (formation of a gas)?
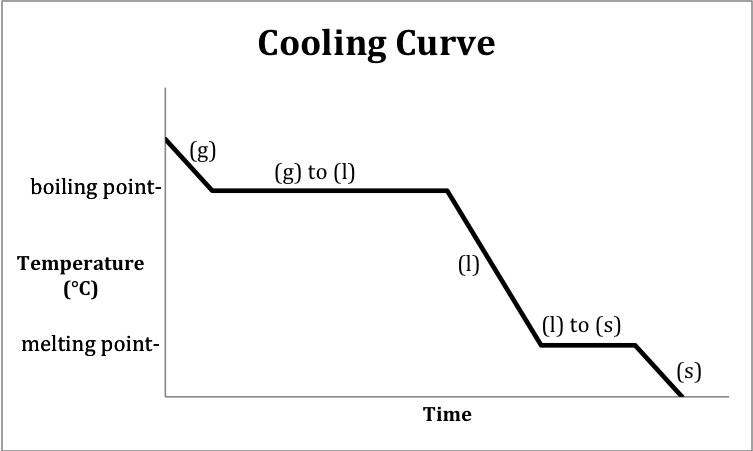 This is the phase change occurring from l to s
This is the phase change occurring from l to s
What is freezing?
This is the temperature (in Celsius) at which water undergoes the freezing/melting process.
0 degrees Celsius
If an object had a volume of 3.7 mL and a density of 11.3 g/mL, what is its mass? (units and SFs)
42g
This is the difference between a physical and chemical property
What is a chemical property will involve a material's ability to form new substance , while a physical property is used to identify a substance without changing its composition?
These are three signs of a chemical change:
What is
a) change in color
b) formation of a gas
c) formation of a precipitate
d) change in odor
e) change in heat or light
Where in this graph is kinetic energy the LOWEST?

What is 1 (the solid state)?
This phase of matter (one of the three common phases) has the greatest kinetic energy and least attractive forces
What is a gas?
Record the measurement to the correct amount of significant figures.
/meniscus02-58b5b2ee5f9b586046bb3f18.png)
6.60 mL (underlined number may vary)