Lightning is largely comprised of which subatomic particle?
Electrons
A force value that is negative represents which type of interaction; repulsion or attraction?
Attraction
Which contribution to atomic theory is Democritus credited with?
Conceptualizing the atom ("atomos")
If the size of q2 increases by 20 times, how much would the force value increase by? Assume no other variable is changing.
Force would increase by 20 times
Lightning generally originates from a negatively-charged region and will strike a positively-charged region. What are some materials that carry positive charges and would be able to attract lightning?
Metal or the ground
Coulomb's Law describes force in terms of which 2 properties?
Charge size and distance between charged regions
Which subatomic particle did JJ Thomson discover?
The electron
If the distance between 2 charged areas decreases by 10 times, how would the force value change? Assume all other variables remain the same.
Force would increase by 100 times
In the provided image, areas that are highlighted in red demonstrate the greatest amount of lightning strikes on the planet. What do these locations have in common?
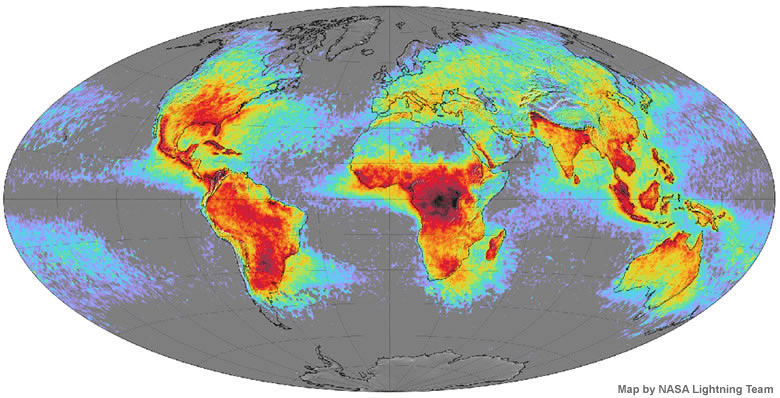
Tropical (tend to be hot and humid) and mostly near the equator
Based on the Coulomb's Law equation, what are the units tied to the variable "k?"
Nm2/C2
The provided diagram represents which scientist's experiment? (hint: this experiment resulted in the discovery of a positively-charged nucleus)
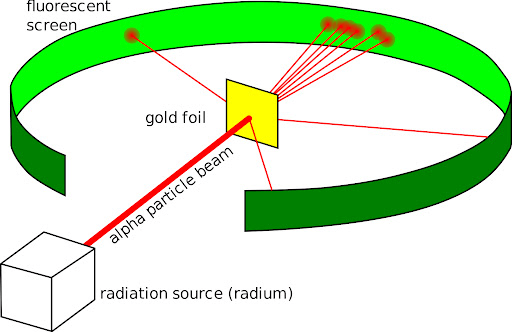
Ernest Rutherford
If q1 has a charge of -4 mC, q2 has a charge of +4 mC, and the distance between the charges is .2 m, what is the force value?
-3.60 x 106 N
How are positive and negative regions developed in a cloud within a lightning storm? Be specific.
Water molecules in the clouds collide with one another and transfer electrons. Some water molecules gain electrons (become more negative) and some water molecules lose electrons (become more positive). This is the general concept behind static electricity.
How would you arrange the following equation to solve for "k?"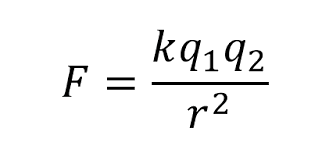
k = Fr2/q1q2
The provided image represents a Bohr model of an atom. How would Schrodinger's model differ?
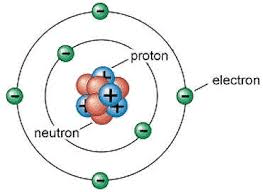
The spacing/size of electron shells would not be increasing by small increments (would be more of an exponential increase in shell size)
If q1 has a charge of -1.46 uC, q2 has a charge of +2.32 mC, and r has a value of 213 cm, what is the force value?
-6.71 N
Electricity, like water and many other substances, moves throughout an environment based on the path of least resistance. What does the path of least resistance mean?

Lightning and other substances will move throughout the environment where there are the least obstructions in its way. Basically, fluids, gases, particles, etc. want to move in a way that allows them to get from point A to point B the fastest
Coulomb's Law not only describes interactions between charged areas on a larger scale, but also at the particle level as well. If the differences in charge between particles are rather low, do you think this would likely result in a polar or non-polar molecule?
Likely non-polar
Of Dalton's 4 principles regarding atomic properties, which of the 4 was Dalton's only true unique contribution towards atomic theory?
Atoms combine in simple ratios to form molecules
If q1 has a charge of +15.2 uC, q2 has a charge of +13.1 uC, and the force value is 776 mN, what is the distance between the charged regions?
1.52 m