The number of significant figures in 0.0075 g
2
10.4168 m - 6.0 m =
4.4 m
What place value and unit would this ruler read to?

100ths place
X.XX cm
Why does a bubble of carbon dioxide rise to the top of a glass of soda?
The density of the carbon dioxide is less than the density of water
What kind of specific heat capacity do you have if you do not change your temperature readily?
High specific heat
The number of significant figures in 150.0 mL
4
1.31 cm x 2.3 cm =
3.0 cm2
What place value and unit would this graduated cylinder read to?
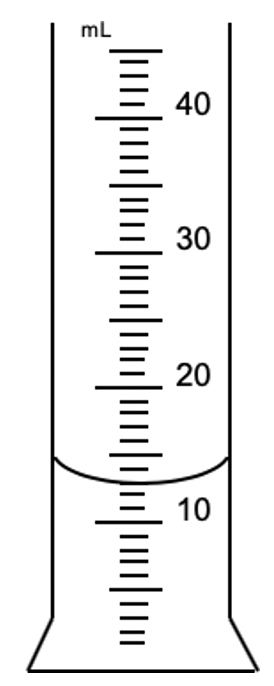
10ths place
XX.X mL
The 2nd most dense solid

Popcorn Kernel
Which substance would increase temperature the fastest?
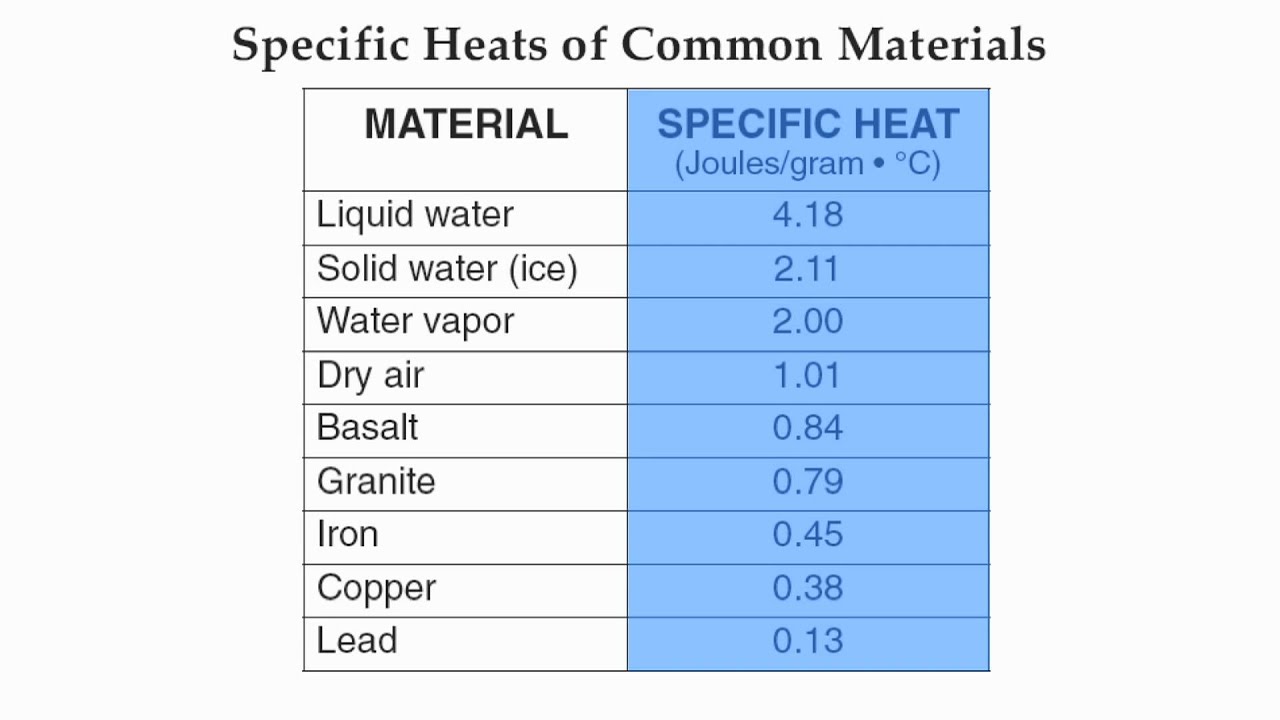
Lead
Round 0.0583 mm to 1 significant figures
0.06 mm
20.2 cm / 7.410 s =
2.73 cm/s
What is the length of the object below?
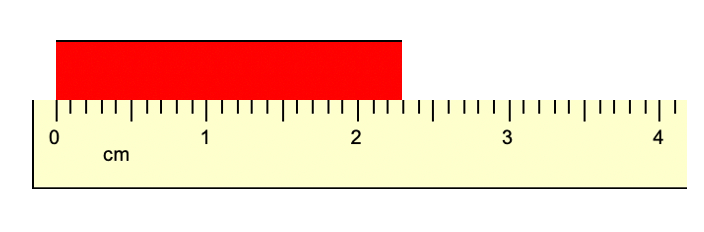
2.29 cm
or
2.30 cm
Use the data below to calculate the density of silly puddy

2 g/mL
What type of substances have low specific heats?

Metals
Round 1864.2 km to 2 significant figures
1900 km
6.201 in. + 7.40 in. + 12.0 in. =
25.6 in
Determine the volume of the liquid in the graduated cylinder.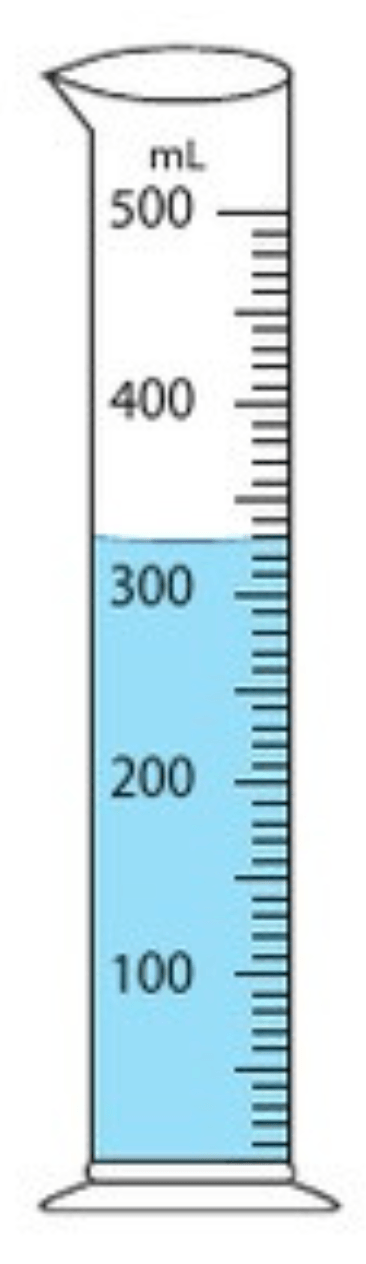
328 mL
(327 - 330. would be acceptable)
A block of copper metal has a mass of 1896 g. The dimensions of the block are 8.40 cm x 5.45 cm x 4.6 cm. What is the density of copper?
9.0 g/cm3
Which substance would increase temperature the slowest?

Liquid water
Round 0.05697 L to 3 significant figures
0.0570 L
5.7621 m x 6.901 m x 0.460 m =
18.3 m3
Read the temperature off of the thermometer

19 °C
A flask with a mass of 345.8 g is filled with 225 mL of carbon tetrachloride (CH4). The mass of the flask and CH4 is found to be 703.55 g. Calculate the density of CH4
1.59 g/mL
What is the definition for specific heat capacity?
Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (°C).