This subatomic particle that determines an element's identity.
protons
The atomic number of this atom: chlorine-35
What is 17?
What do isotopes have in common and what differs between them?
Different: neutrons, mass number
The order of radiation from least penetrating to most.
alpha, beta, gamma
This color has the shortest wavelength of visible light
violet
A particle that carries a charge is called what?
An ion
+=cation
-=anion
The mass, charge, and location of each subatomic particle.
n0: 1 amu, 0, in the nucleus
e-: 0 amu, 1-, in the electron cloud
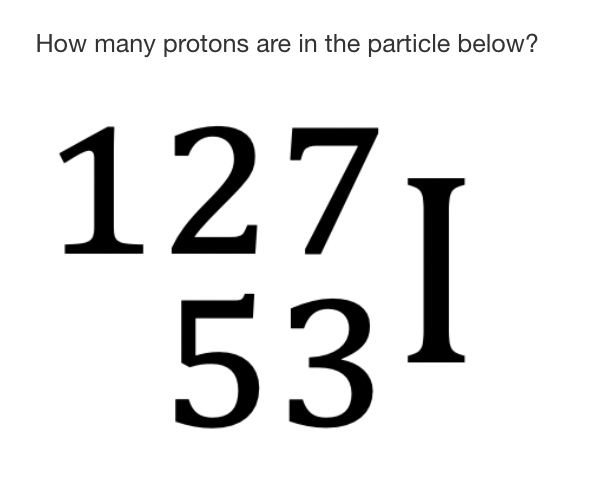
53
Lithium has two naturally occurring isotopes: lithium-6 and lithium-7. If the average atomic mass of lithium is 6.941 amu, which isotope is the most abundant?
Li-7
Does the following reaction represent fission or fusion?
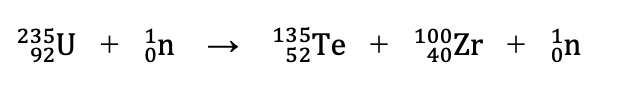
Fission
Which has a higher energy: radio waves or infrared?
infrared
In order to form a positive ion, what must occur?
Lose 1 or more electrons
The number of protons, neutrons, and electrons in the following ion
iron-56
p+:26
n0: 30
e-: 26
Total number of electrons in the following ion:
What is 10?
Estimate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32 amu, 0.76% have a mass of 33 amu, and 4.22% have a mass of 34 amu.
a little above 32 amu (32.02-32.3)
Does the reaction below represent fission or fusion?

Fusion
Light with a _________ wavelength has more energy
shorter
What causes an isotope to be radioactive?
The number of protons, neutrons, and electrons in the following ion
magnesium-25 2+
p+:12
n0: 13
e-: 10
The total subatomic particles in nucleus:

What is 63?
Calculate the average atomic mass of Iridium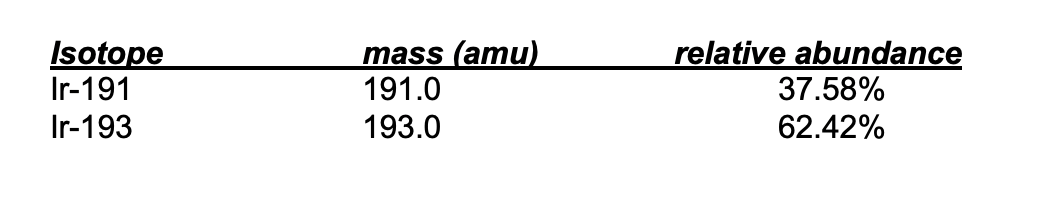
What is 192.2 amu?
What goes into the blank?

42He
When does an atom release energy in the form of light?
When an electron moves from a higher energy level (excited state) to a lower energy level.
Atoms are electrically _____________.
Neutral
Put the following atomic model in chronological order

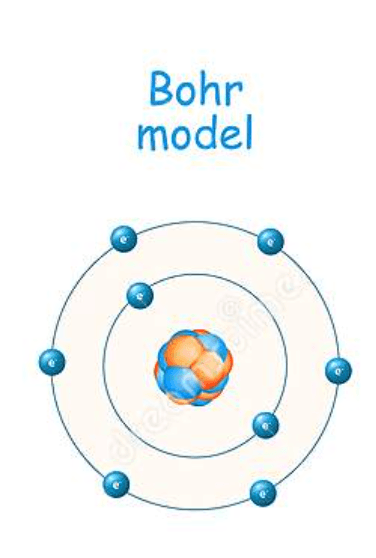


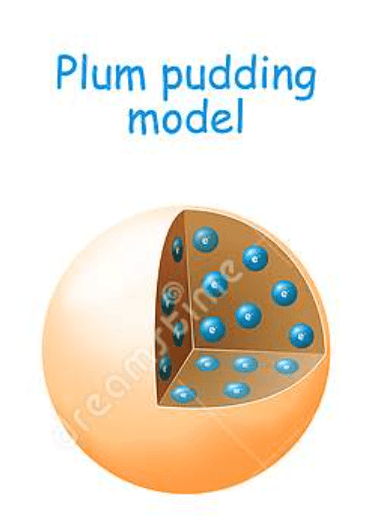
Billiard Ball
Plum Pudding
Planetary (Rutherford)
Bohr
Electron Cloud (most current)
Total subatomic particles:

What is 32?
Titanium has five common isotopes: 46Ti (8.0%), 47Ti (7.8%), 48Ti (73.4%), 49Ti (5.5%), 50Ti (5.3%). What is the average atomic mass of titanium?
47.92 amu
What goes in the blank?
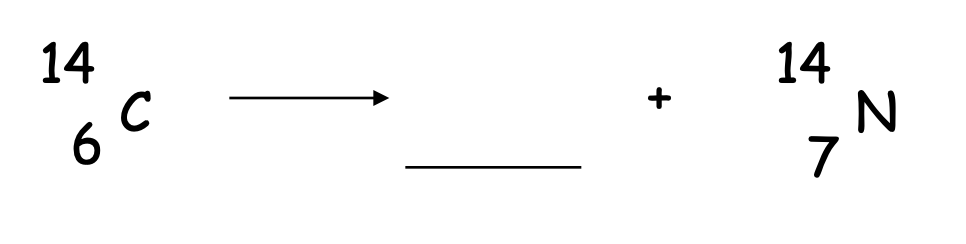
0-1e
Which elements make up the mixture based on the emission spectra shown below
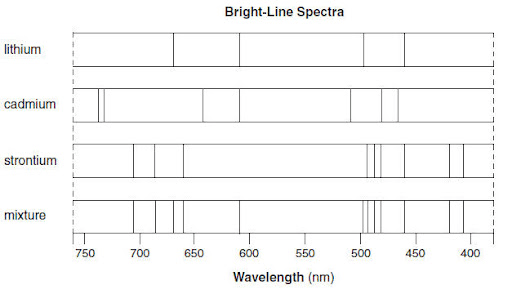
lithium & strontium
How can you tell that an element has no stable isotopes?
The atomic mass of the element is listed in parenthesis on the periodic table