Based on the following chemical model below, the farthest circle (third energy level) contains what kind of electrons?
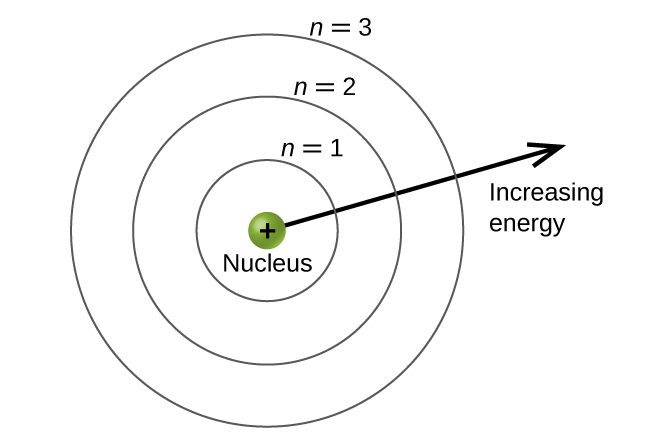
Valence Electrons
In order for a covalent bond to form, the valence electrons will have to _______________
be shared by two or more atoms
Lewis dot structures only show these types of electrons
valence electrons
intermolecular forces exist between ________ compounds.
covalent
name this compound:
N2O4
dinitrogen tetroxide
How can you tell if a compound is ionic?
If the compound contains a metal and a nonmetal, this is considered as an ionic compound.
draw the Lewis structure for water

order the 3 intermolecular forces we learned about from weakest to strongest
London Dispersion Force, Dipole-Dipole, Hydrogen Bonding
What is the charged of a Barium ion in order for it to form ionic compounds?
Barium has a 2+ charge (Ba2+)
How does an ionic compound conduct electricity?
If the ionic compounds are melted or dissolved, they will conduct electricity due to the presence of ions.
The following diagram shows the transfer of electrons to form what ionic compound? (Please provide the chemical name/formula)
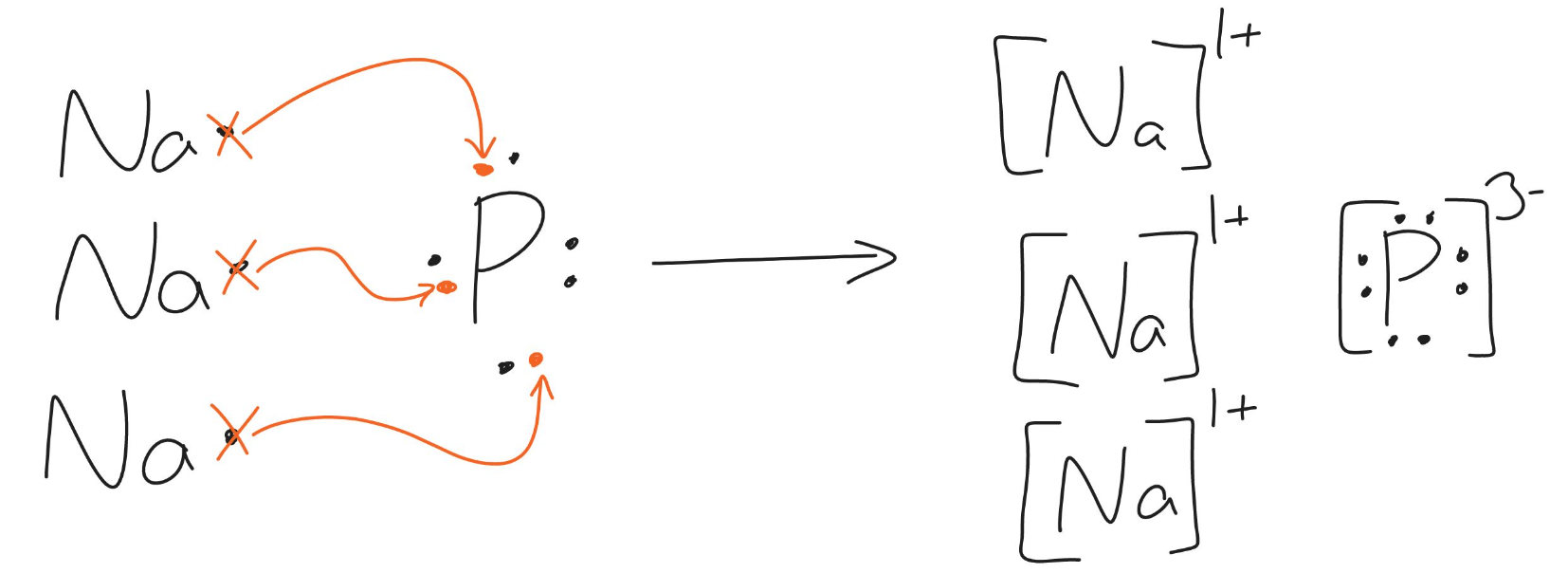
Sodium Phosphide (Na3P)
fluorine, oxygen, and nitrogen are 3 elements that are necessary for the formation of these IMFs
hydrogen bonds
name this compound: Sn(CO3)2
Tin (IV) Carbonate
write the chemical formula potassium phosphide
K3P
Draw the lewis dot structure for CaCl2
This is an ionic compound, therefore, you will need to include brackets on the drawing.
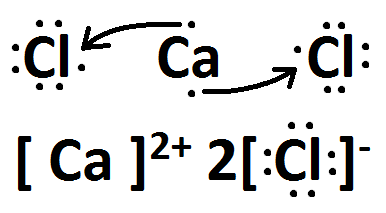
CH3Cl is considered to be a polar covalent molecule, what is the strongest intermolecular force does this molecule contain?

This molecule has dipole-dipole and London dispersion force, however, the strongest force this has is dipole-dipole.
name this compound: P2Cl5
Diphosphorus Pentachloride
Provide one example of an ionic and molecular compound.
Ionic: Metal and a nonmetal
ex. NaCl, CaBr2, etc
Covalent: Nonmetal with a Nonmetal
ex. CO2, CCl4
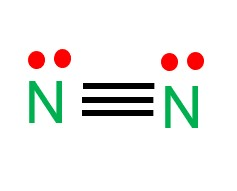
Draw the lewis structure for a nitrogen molecule (N2) and determine its shape
Linear
How do you know if a molecule is considered to be polar or nonpolar?
If the electrons are shared unequally in a molecule then it will be polar.
However, if the electrons are shared equally in a molecule, then it will be considered as nonpolar