A substance that cannot be broken down any further than it is; for example, oxygen or calcium
What is an element?
A covalent bond forms between
Two nonmetals
This is either a bond or a lone pair of electrons
What is an electron domain?
What is the name for for MgO?
What is magnesium oxide?
This type of ion has a positive charge and form from metals.
What is a cation?
Examples include H2O, CO2, and NaCl
What is a compound?
Which of the following compounds is ionic: KCl or HCl
What is KCl?
If a compound has two electron domains, it takes this shape.
What is linear?
In naming a covalent compound, we must add these to the beginning of element's name.
What are prefixes
This type of ion has a negative charge and forms from nonmetals.
What is an anion?
A mixture in which the components are evenly mixed, such as lemonade or Kool Aid
What is a homogenous mixture?
An ionic bond involves this action with electrons.
What is transferring or giving away electrons?
Water has this kind of molecular geometry.
What is bent?
Name CoI2
What is cobalt(II) iodine
Out of solids, liquids, and gases, this state of matter has the most energy.
What are gases?
A mixture in which the components are not evenly mixed, such as fruit salad or trail mix
What is a heterogenous mixture?
Which of the following compounds is ionic? SrO or CO2
What is SrO?
 _________________ is the molecular geometry.
_________________ is the molecular geometry.
What is trigonal pyramidal?
When naming an ion of a transition metal, the numerical value of the charge is indicated by what?
What is a Roman numeral?
This many atoms are in the formula, C6H12
What is 18 atoms?
The fourth image shows this classification of matter.
What is an element?
This type of bond involves unequal sharing of electrons
What is a polar covalent bond?
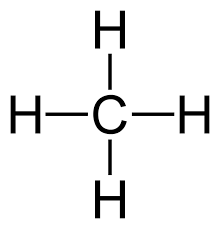 ________________ is the molecular geometry.
________________ is the molecular geometry.
What is a tetrahedral?
The name for Ca3P?
What is calcium phosphide?
Given the ideal gas law, PV = nRT, solve for T.
The pressure is 1 atm, the volume 4 L, n =2 moles, and R=0.08206
What is 24.39 K?