The element in the bottom right of the periodic table, Og.
What is the heaviest element?
OR
What is the most massive element
Melting an ice cube is an example of this change
What is a physical change?
This math will answer that question about Na2S.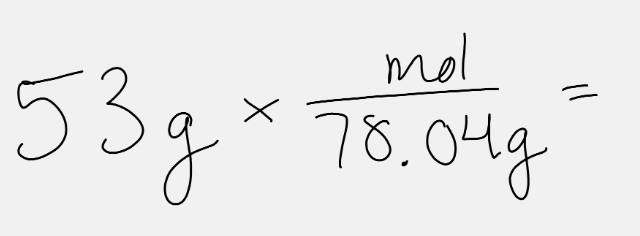
What are the moles in 53 g of Na2S?
Based on the chemical equation 2 H2O --> 2 H2 + O2, this math will answer the question.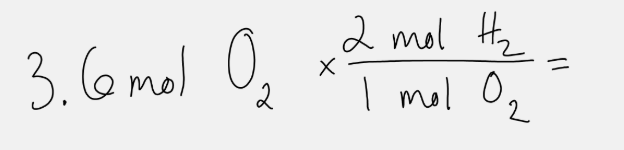
What are the moles of H2 made if 3.6 moles of O2 are made?
This diagram is showing an example of this type of substance
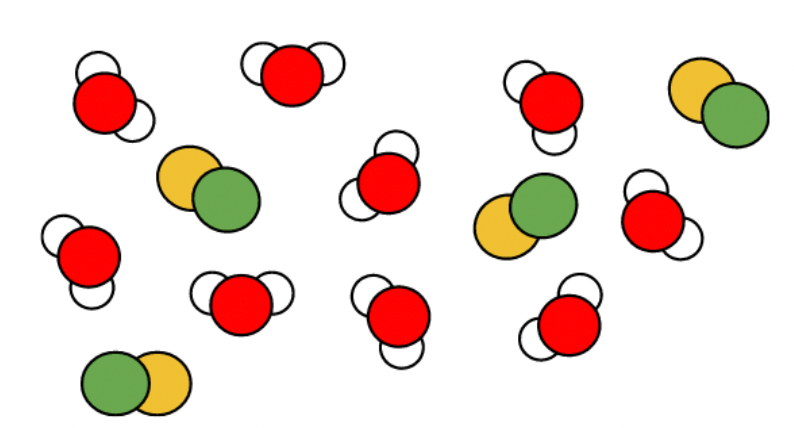
What is a mixture?
This diagram represents that type of change
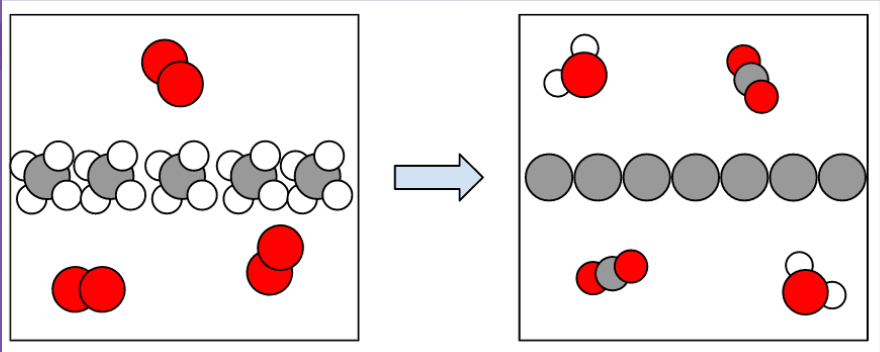
What is a chemical change?
This diagram shows that chemical formula.
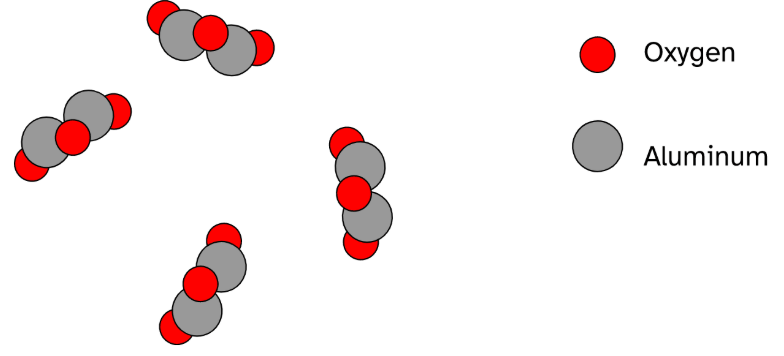
What is Al2O3?
Based on the reaction 2 H2 + O2 --> 2 H2O, this is the moles of water I can produce with 5 moles of O2
What is 10 moles?
This is the best method for separating a mixture of A, B, and C.
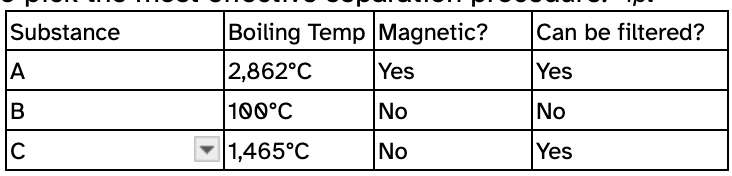
What is filtration and using a magnet?
Based on the heating curve of hexane, we know that this substance will vaporize first in a mixture of hexane and water.
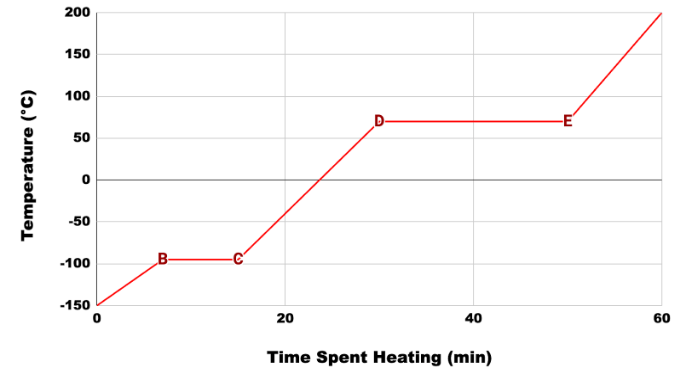
What is hexane?
Based on the chemical formula, this math would answer the question.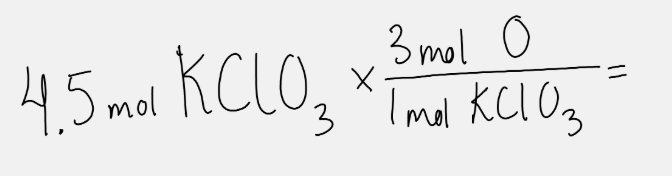
What are the moles of O in 4.5 moles of KClO3?
These are the coefficients for the chemical equation:
__ Zn + __ HCl --> __ ZnCl2 + __ H2
What is 1 Zn + 2 HCl --> 1 ZnCl2 + 1 H2 ?
These are 2 things that will be different for 2 6 mole samples, one of a diatomic element and one of a nondiatomic element.
What are the number of separate formula units AND the volume?
This is the complete balanced chemical equation for solid magnesium reacting with gaseous diatomic oxygen to produce solid magnesium oxide (MgO).
2 Mg(s) + O2 (g) --> 2 MgO (s)
For a sample with 3.8 g of S and 13.7 g of F, this math will answer this question.
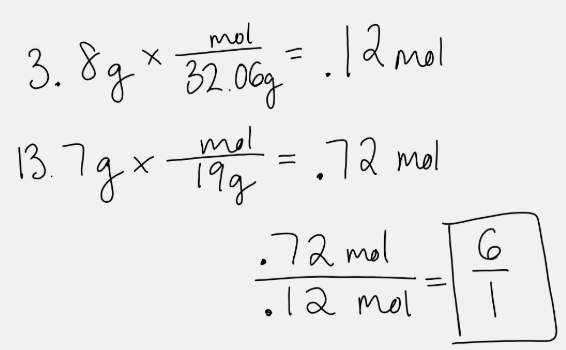
What is the chemical formula?
OR
What is SF6?
Based on the reaction 2 H2 + O2 --> 2 H2O, this is the moles of water I can produce with 32 g of O2