What is the main carbohydrate product of the photosynthesis life cycle, and what is its chemical formula?
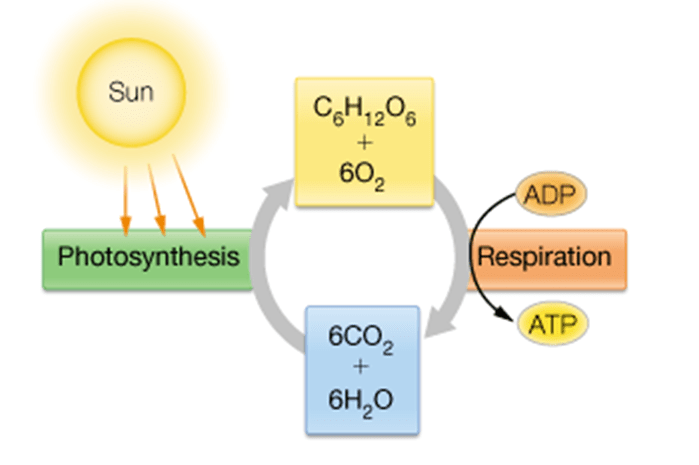
The main carbohydrate is glucose, with the formula C₆H₁₂O₆.

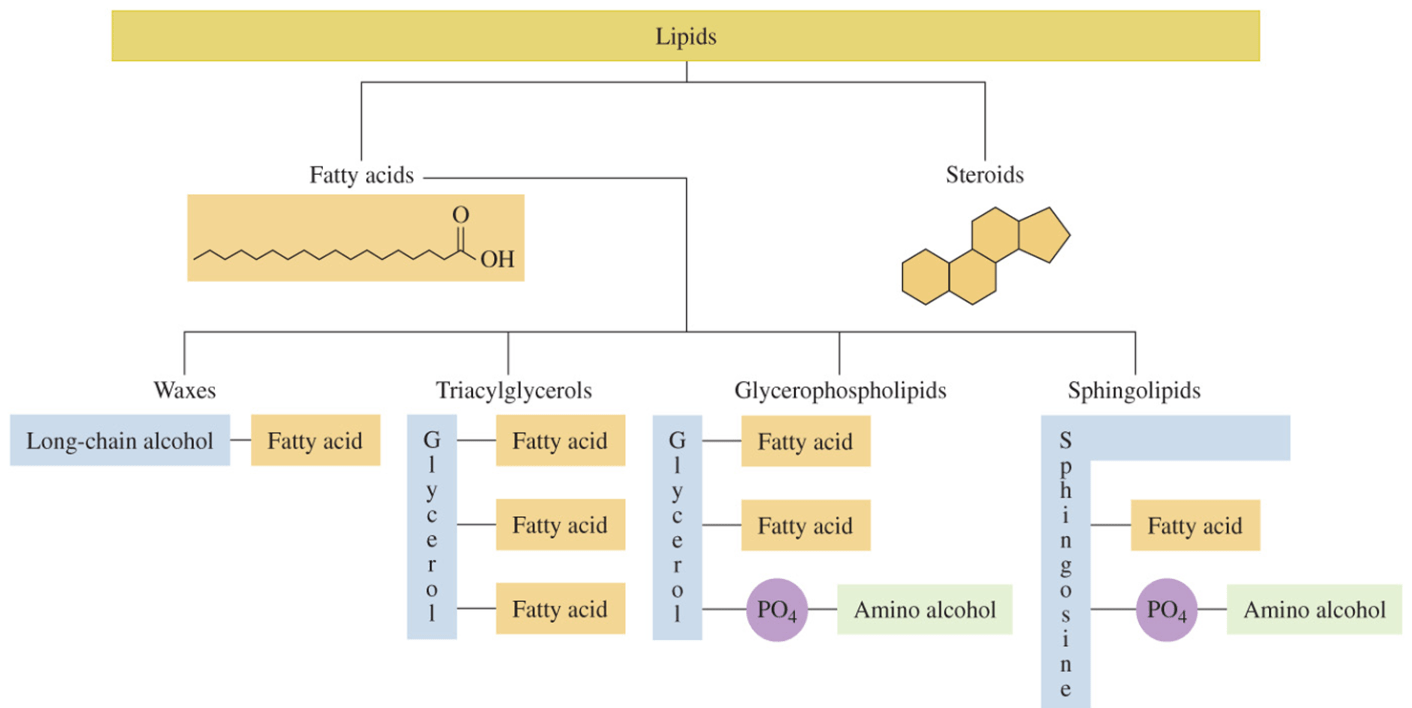
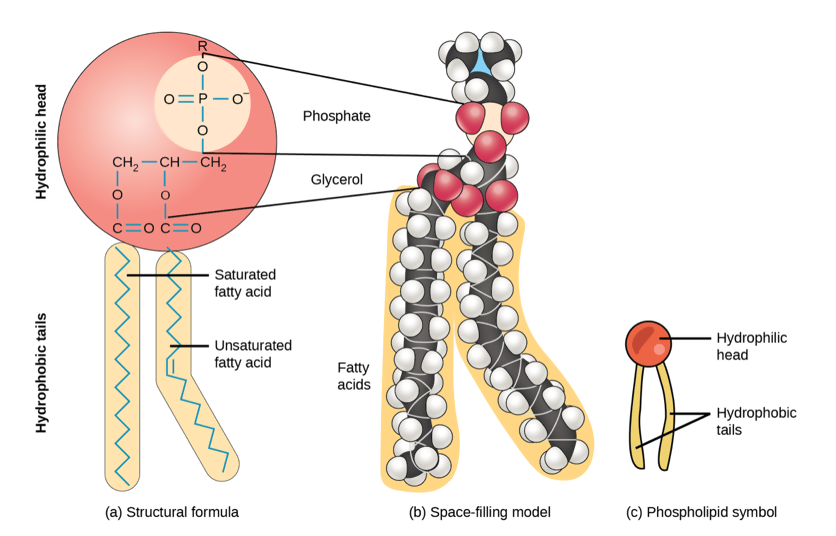
How do you distinguish between the structures of a phospholipid, triacylglycerol, and steroid?
Phospholipid: 2 fatty acids + glycerol + phosphate group (often with a head group like choline).
Triacylglycerol (TAG): 3 fatty acids + glycerol (no phosphate group).
Steroid: Four fused rings (3 six-membered, 1 five-membered), no fatty acid tails.
What is metabolism?
Metabolism is the sum of all chemical reactions in a cell, including both:
Catabolism (breaks down molecules, releases energy)
Anabolism (builds molecules, uses energy)
What is the difference between monosaccharides, disaccharides, polysaccharides, and starch?
Monosaccharides: Single sugar units (e.g., glucose, fructose).
Disaccharides: Two monosaccharides linked (e.g., sucrose, lactose).
Polysaccharides: Long chains of many monosaccharides (e.g., cellulose, glycogen).
Starch: A type of polysaccharide made of α-glucose units used for energy storage in plants.
What makes a fatty acid unsaturated?
A double bond between carbon atoms in the hydrocarbon chain (C=C) introduces a "kink" and reduces tight packing.

What do aerobic, anaerobic, heterotroph, and autotroph mean?
•Autotrophs – photosynthetic organisms get their energy from the sun to synthesize glucose and all of their other organic compounds from inorganic CO2
•Heterotrophs – get energy through oxidation of food generated by phototrophs to synthesize their organic metabolites
•Aerobic organisms – depend on aerobic respiration to get their energy in the presence of oxygen; most multicellular organisms and many bacteria
•Anaerobic organisms – can get energy in the absence of oxygen
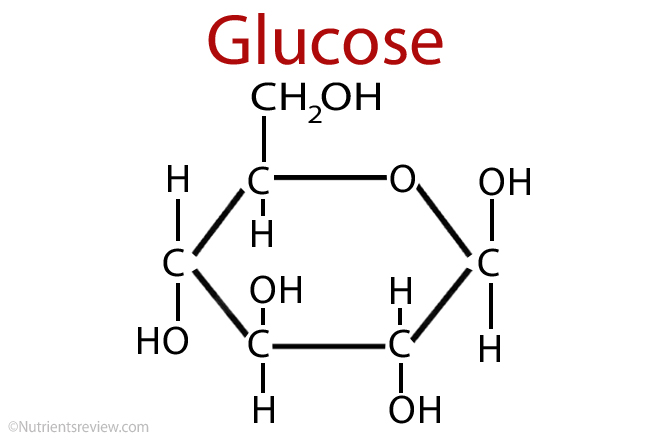
Chemically describe and classify glucose. Is it L or D? Aldose or ketose? Reducing or non-reducing?
Glucose is a D-aldose (D-configuration, contains an aldehyde group). It is a reducing sugar because it has a free anomeric carbon in its open-chain form.
What is the key distinction between sphingolipids and phospholipid
Phospholipids are built from glycerol, have 2 fatty acids + phosphate.
Sphingolipids are built from sphingosine, have 1 fatty acid + phosphate or sugar group.
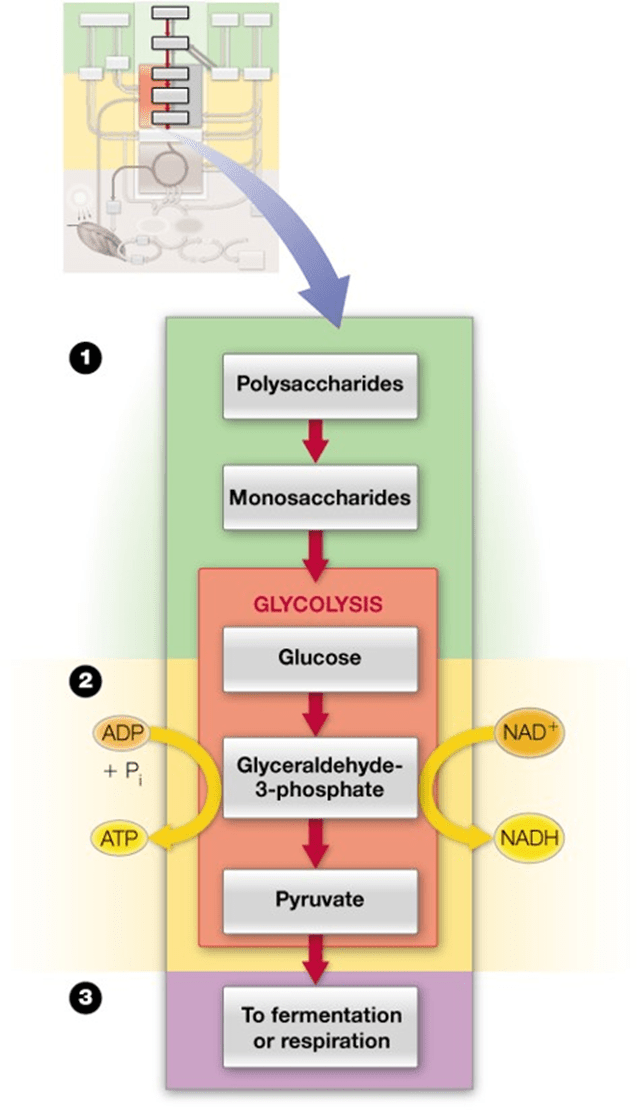
What is glycolysis?
A metabolic pathway that breaks down glucose (C₆H₁₂O₆) into 2 pyruvate molecules, producing ATP and NADH.
•Glycolysis is the initial phase of carbohydrate metabolism
•Major input is glucose derived from mono-, di-, and polysaccharides
•Major output is pyruvate
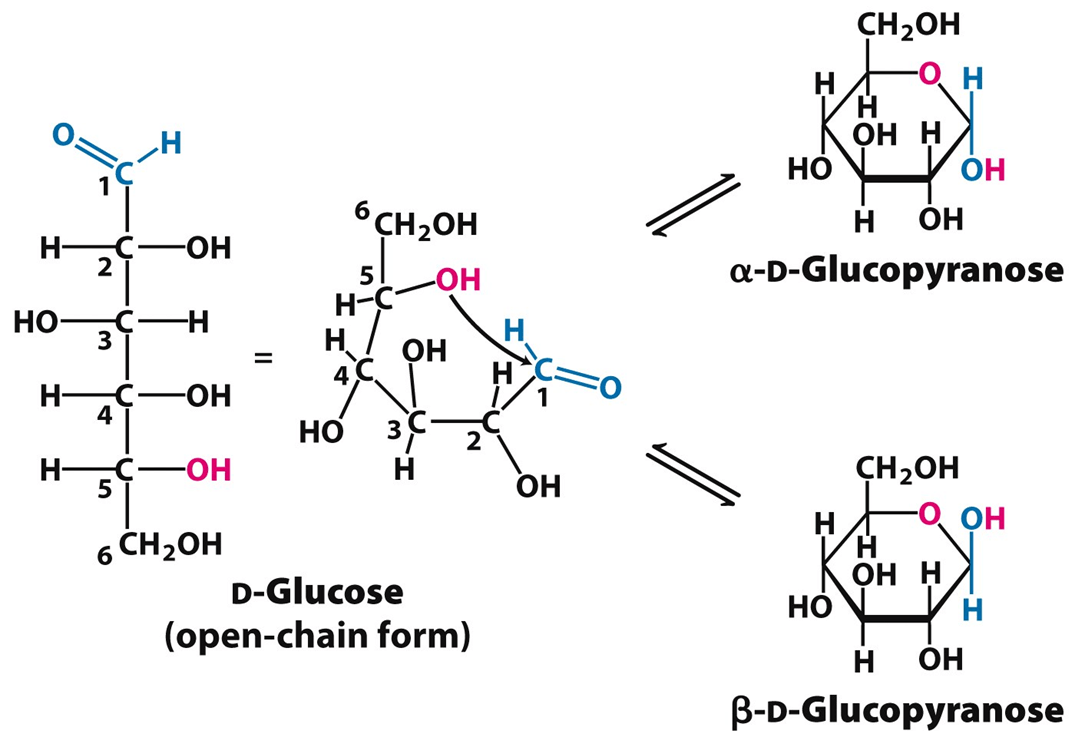
What is the anomeric carbon in a cyclic monosaccharide, and how do you identify it?
The anomeric carbon is a specific carbon atom in a cyclic carbohydrate that was originally the carbonyl carbon (aldehyde or ketone) in the acyclic (linear) form.
What is the fluid mosaic model of cell membranes?
The fluid mosaic model describes membranes as a fluid bilayer of phospholipids with proteins embedded or floating, allowing lateral movement and dynamic structure.
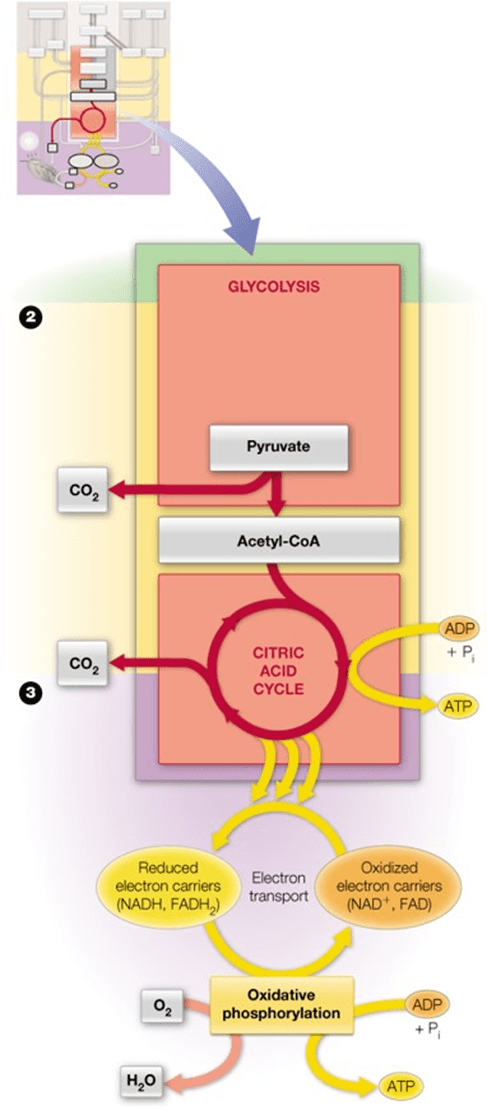
What is the citric acid cycle?
A cyclic metabolic pathway that oxidizes acetyl-CoA to CO₂, generating NADH, FADH₂, and ATP.
•Pyruvate to acetyl-coenzyme A (acetyl-CoA) is the bridge from glycolysis to the citric acid cycle
•Pyruvate undergoes oxidative metabolism (respiration) as it is oxidized in the presence of oxygen to acetyl-CoA
•The citric acid cycle (CAC) accepts acetyl-coA as the input from carbohydrates, lipids, and amino acids
•Major output is NADH and FADH2
•Notice CO2 is also given off
•All catabolic processes converge at the CAC
How can you identify the oxidized form of glucose when given a structure?
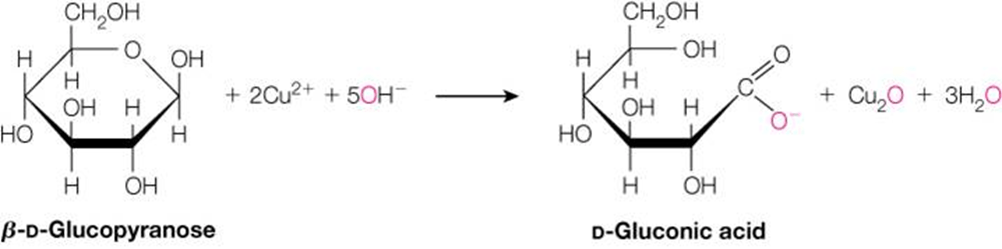
The oxidized form has a carboxylic acid group (COOH) instead of an aldehyde (CHO). This form is called gluconic acid when the aldehyde is oxidized.
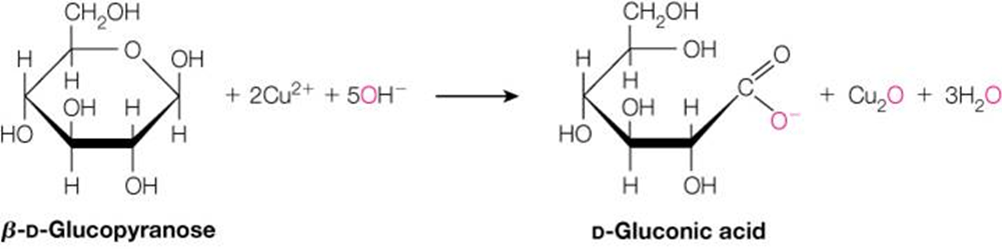
What’s the difference between active and passive transport? Give examples.
Passive transport: No energy, moves substances down concentration gradient (e.g., diffusion, facilitated diffusion).
Active transport: Requires energy (usually ATP), moves substances against the gradient (e.g., Na⁺/K⁺ pump, proton pump).
What is oxidative phosphorylation?
The process by which ATP is produced using electron transport chain and proton gradient across the mitochondrial membrane, involving O₂ as the final electron acceptor.
•In oxidative phosphorylation, the NADH and FADH2 are oxidized to drive the synthesis of ATP
•The major output is water and ATP
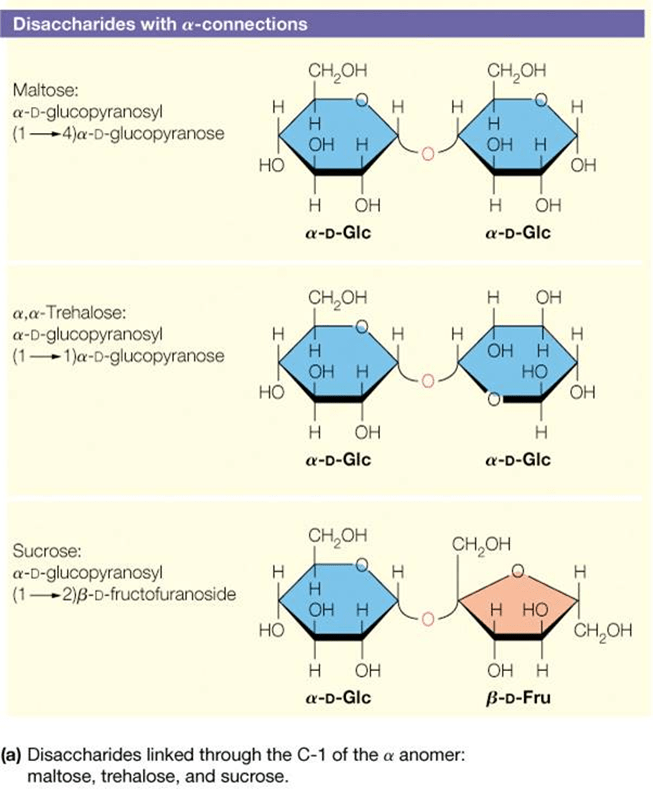
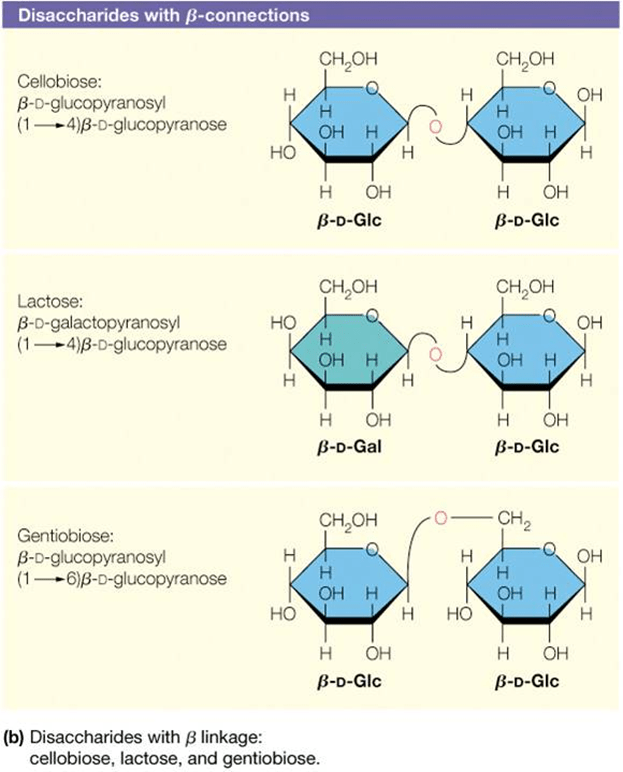
How do you identify the glycosidic bond in a disaccharide structure?
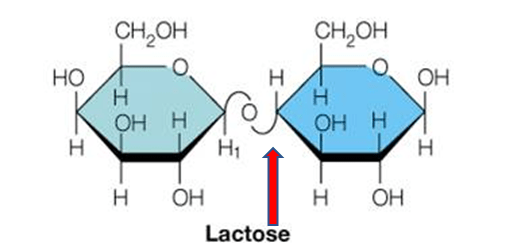
Glycosidic bonds form between the anomeric OH group of one cyclic monosaccharide and an additional group. OH is lost as water
Look for the oxygen bridge connecting two sugar rings.
Identify the carbons involved (e.g., α(1→4) or β(1→6)).
The anomeric carbon of at least one sugar is involved in the bond.
In the equation for diffusion, what do the variables mean?
T = temperature. Assume body temperature if not given = 310 K
R = ideal gas constant = 0.008314 k J/ mol K
Z = ionic charge (use periodic table)
F = Faraday’s constant = 96.5 kJ/mol V
∆Ψ = Ψf - Ψi this is the change in membrane potential
What is a nucleophile and electrophile?
Nucleophile: Electron-rich species that donates electrons (e.g., OH⁻, NH₃).
Electrophile: Electron-poor species that accepts electrons (e.g., carbocations, carbonyl carbon).
What are the three main functions of polysaccharides?
1)energy storage polysaccharides (e.g., starch and glycogen)
2)structural polysaccharides (e.g., cellulose)
3)lubricants (e.g., some glycosaminoglycans)
What are some examples of molecules that mediate facilitated transport?
Protein pores (e.g., aquaporins)
Permeases (e.g., glucose transporter, GLUT)
Ionophores (e.g., valinomycin carries K⁺ across membrane)
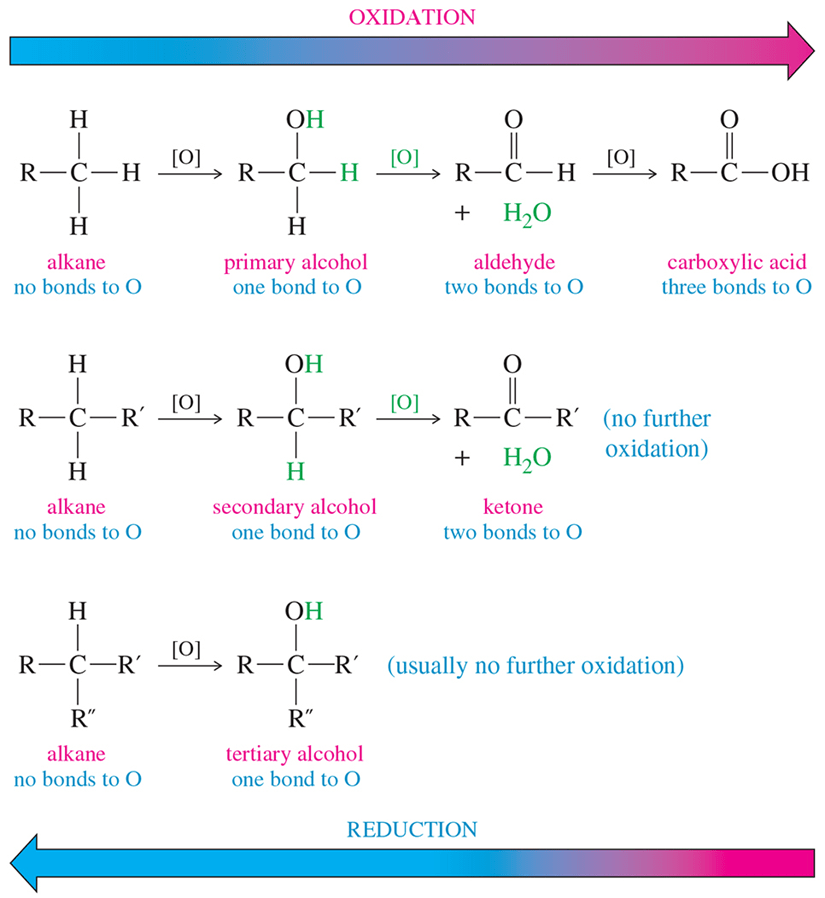
How is oxidation and reduction defined biochemically?
Oxidation = loss of e-
= gain bonds to O
= fewer bonds to H
Reduction = gain of e-
= gain bonds to H
= fewer bonds to O
What is the main difference between cellulose and amylose in terms of glycosidic bonds?
Cellulose has β(1→4) glycosidic bonds (straight, rigid structure).
Amylose has α(1→4) glycosidic bonds (forms helical structure).

What are P-type ATPases and ABC transporters?
P-type ATPases: Pumps that get phosphorylated during ion transport (e.g., Na⁺/K⁺ pump).
ABC (ATP-Binding Cassette) transporters: Use ATP binding/hydrolysis to transport molecules across membranes (e.g., multidrug resistance protein).
How many ATP molecules are produced from the complete oxidation of one glucose molecule in cellular respiration?
Approximately 30 to 32 ATP molecules are produced from one glucose molecule.
What are glycosaminoglycans, and what do they do?
•Play structural and nonstructural role in vertebrates
•Important part of skin, connective, epithelial and neural issues
•Some act as lubricants in mucus and other body fluids
•Some function as anticoagulants (heparin)
•Polymers of repeating disaccharide units
How does a P-type ATPase pump against a concentration gradient?
It uses ATP hydrolysis to change its conformation and actively move ions, like Na⁺ out and K⁺ in, against their gradients.
What does the sign of Gibbs free energy (ΔG) tell you about a reaction?
ΔG < 0: Reaction is spontaneous and favorable
ΔG > 0: Reaction is nonspontaneous and unfavorable
What is a glycoprotein, and how are sugars connected to proteins?
A glycoprotein is a protein with carbohydrate (sugar) groups covalently attached.
•Glycans are linked in 2 ways:
1.N-linked to the side chain of the amino acid asparagine
2.O-linked to the side chain of the threonine or serine amino acids
How does the sodium-glucose cotransport system work?
In cotransport, the unfavorable movement of a substance through a membrane is coupled to the favorable transport of another substance
In the sodium-glucose cotransport system, the sodium gradient across the membrane provides the driving force for the unfavorable transport of glucose
What are some major mechanisms of metabolic control?
•Control of enzyme concentration through the regulation of enzyme synthesis and degradation
•Enzyme activity can be controlled by altering substrate concentration, allosteric controls, and covalent modification
•Metabolite concentration is controlled by compartmentation
•Hormones act at all levels of regulation often using second messengers such as cAMP