An electron absorbs 9.8 electron volts of energy. How many electron volts will it release when it goes back to the ground state?
9.8 electron volts
CP: What element is 1s2 2s2 2p6 3s2 3p2?
Honors: What element is this?
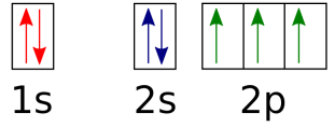
CP: Silicon
Honors: Nitrogen
Name SrO
Strontium Oxide
What is the formula for francium sulfide?
Fr2S
When are eClass quizzes due?
DAILY DOUBLE
Before the test!
What is the volume of the liquid?

36.5 mL (or 36.4 mL or 36.6 mL)
Why are noble gases unreactive?
Full valence shell, octet rule satisfied
Name Rb2CO3
Rubidium Carbonate
Potassium Chloride
KCl
Why did the atomic model change over time?
New evidence/new discoveries
Group the isotopes together
a) 3 protons 4 neutrons
b) 3 protons 3 neutrons
c) 4 protons 4 neutrons
d) 3 protons 5 neutrons
e) 4 protons 5 neutrons
A,B,D are a group of isotopes
C and E are a group of isotopes
(same protons, different neutrons)
What is the name of the group that has their p-sublevel filled?
Al(OH)3
Aluminum Hydroxide
Vanadium (IV) Oxide?
VO2
An element with a higher number of valence electrons will most likely be a...
a) metal
b) metalloid
c) nonmetal
d) polyatomic ion
C) nonmetal
(right side of periodic table)
Calculate the average atomic mass
Isotope Mass(amu) Abundance
X-34 34.112 75.00%
X-35 35.009 21.40%
X-36 36.122 3.60%
34.376 amu
What is the trend for atomic radius as you go across the periodic table? As you go down the periodic table?
Decreases across the periodic table
Increases down the periodic table
What is the name for ScCl3?
Scandium (III) Chloride
Gold (II) Silicate
AuSiO3
Which of these is NOT an ionic compound?
a) ZrO
b)BaI2
c)AgCl
d)CS2
d) CS2
two nonmetals
Ionic = one metal one nonmetal
Put these models in order from least current to most current (put the letters in order from least to most current)
A)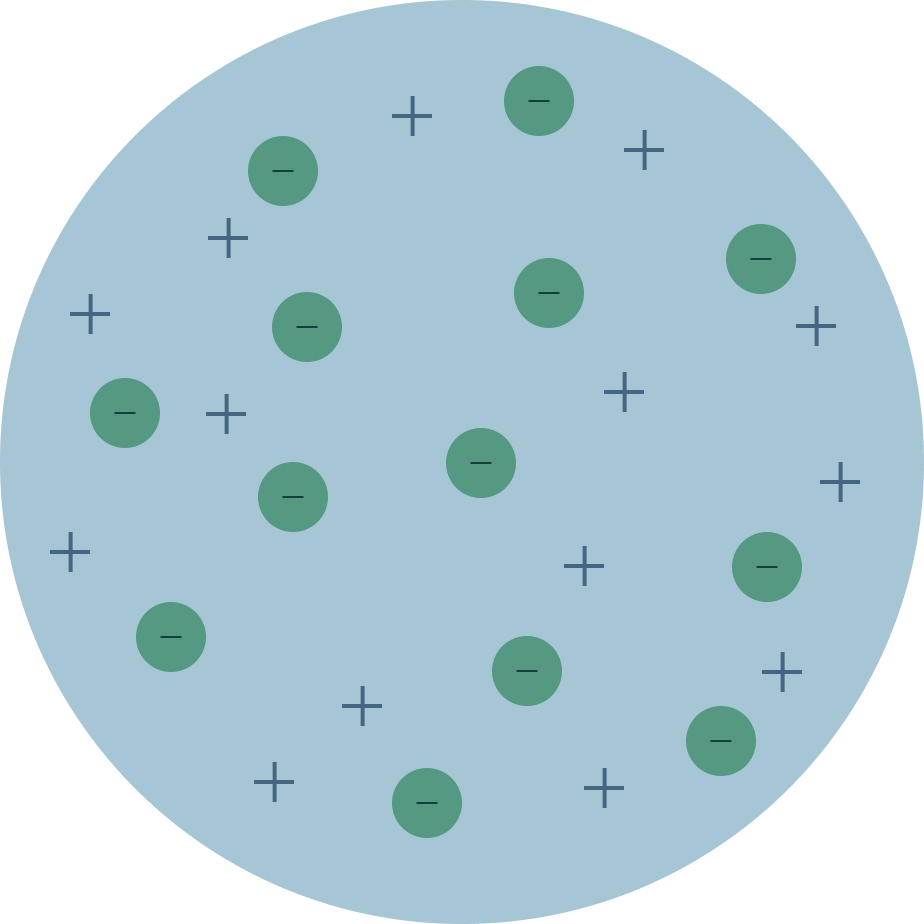 B)
B)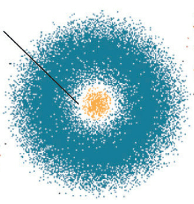
C)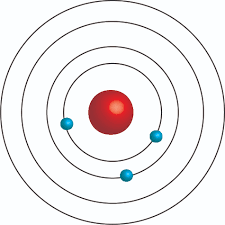 D)
D)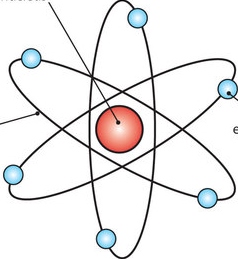
A,D,C,B
Plum pudding, nuclear, solar system, quantum
Explain why potassium and calcium are not likely to react with each other?
Hint: Think about why things react in the first place...
Potassium and calcium are both metals and would need to LOSE electrons in order to satisfy the octet rule
Yttrium (III) Oxalate
What is the formula for aluminum dichromate?
Al2(Cr2O7)3
Explain what is happening in an ionic bond using the words: metal, nonmetal, lose, gain, electron(s)
In an ionic bond, metals lose electrons and nonmetals gain electrons