Average atomic mass is a _________ average
What is the atomic number?
Number of protons in the nucleus
What is c?
Speed of light constant
3.00 * 108 m/s
How many electrons go in the first energy level?
2
The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are listed below. Calculate the average atomic mass of copper.
69.2% for mass of 62.93 amu
30.8% for a mass of 64.93 amu
63.5 amu
Number of protons and neutrons in the nucleus of an atom
Violet light has a wavelength of 4.10 x 10-12 m. What is the frequency?
7.13 * 1019 Hz
How many electrons can go in the second energy level?
8
Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967 amu.
32 amu
Define an isotope
What is the wavelength (in meters) of the electromagnetic carrier wave transmitted by The Sports Fan radio station at a frequency of 640. Hz?
4.69 * 105 m
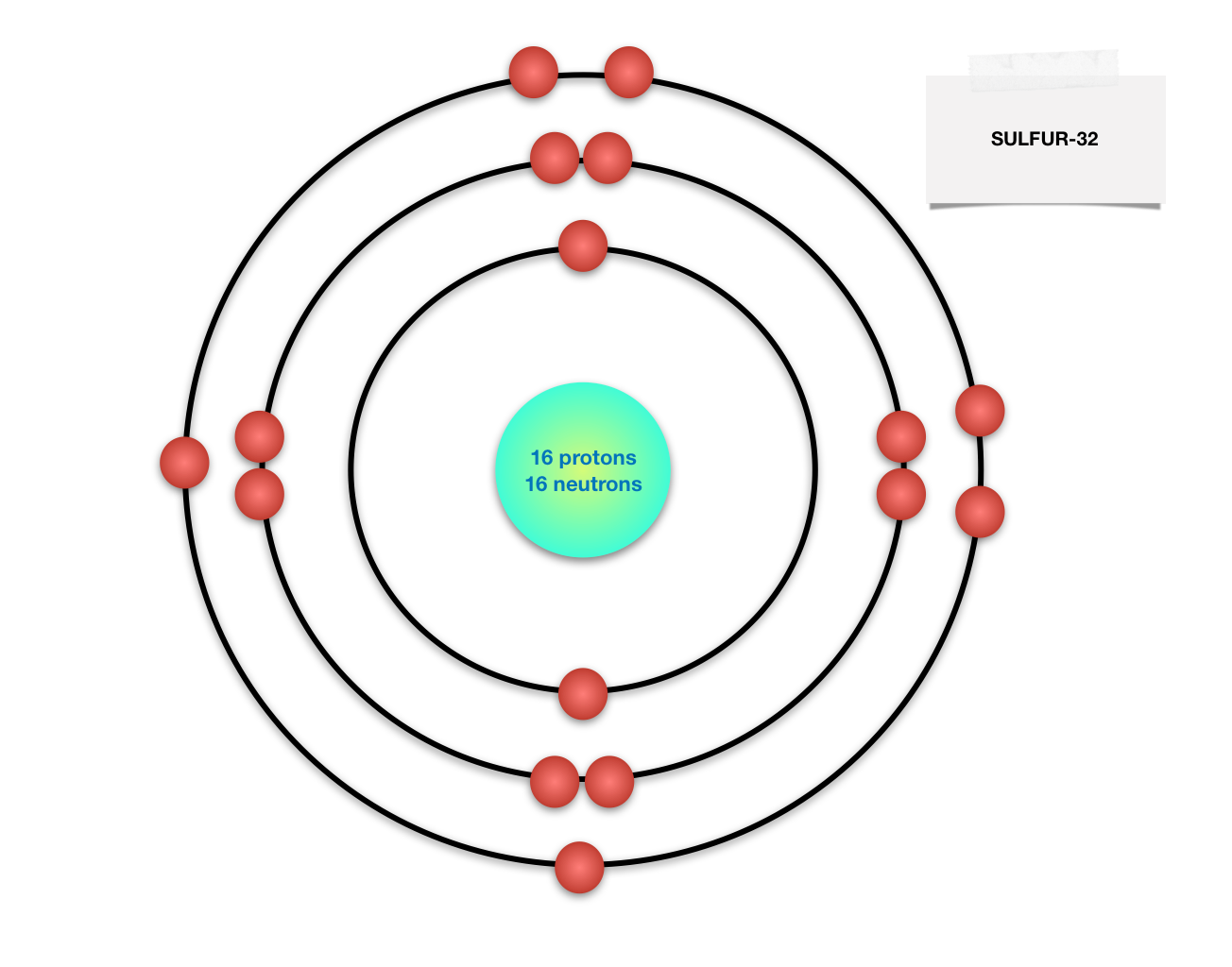
Draw the Bohr model of sulfur-32!
Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium. What is the average atomic mass of strontium?
Sr-84 Sr-86 Sr-87 Sr-88
0.56% 9.86% 7.00% 82.58%
88 amu
Define an ion
Same number of protons, different number of electrons
A helium laser emits light with a wavelength of 633 nm. What is the frequency of the light? (1nm = 1.0 × 10-9 m)
4.73 * 1014 Hz
If an atom is 2 electrons away from completing the second energy level, what will it do to satisfy the octet rule? What will its charge be?
It will gain 2 electrons (charge of -2)
Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92 amu and a relative abundance of 50.69%. The only other major isotope of bromine has an atomic mass of 80.92 amu.
79.90 amu
What is a cation vs anion?
Cation: Positively charged ion
Anion: Negatively charged ion
Green light has a frequency of 6.01 x 1014 Hz. What is the wavelength?
4.99 * 10-7 m
If an atom only has one electron in the outermost energy level, how many electrons will it lose/gain to satisfy the octet rule? What will its charge be?
Lose 1 electron (charge of +1)