What elements generally make an ionic compound?
Metal and Non-metal
Structure for LiF
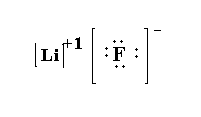
Roman numerals tell you__of the metal cation(s) in an ionic compound?
Charge
Li2O
For binary acids, what prefix do you add in the beginning?
Hydro
Are ionic bonds soluble in alcohol?
No, they are insoluble.
A Lewis Dot Structure is a simplified representation of the ___ ___ in a molecule.
Valence Electrons
What is the name of CO?
Carbon Monoxide
Oxygen Difluoride
OF2
What is the formula for the phosphorous acid?
H3PO3
What's the special rule of Boron?
It is happy with 6 valence electrons.
Draw the Lewis Dot Structure for: NaF

What is the formula for HF?
Hydrogen Monofluoride
What is the formula for ammonium phosphate?
(NH4)3PO4
What is the formula for Nitric Acid?
HNO3
Metallic Bonding occurs between like atoms of a metal in the ___.
Free state
Lewis Dot Structure for BF3

What is the name of C3Cl8?
Tricarbon Octachloride
Formula for Copper (II) Sulfate
CuSO4
If the anion ends in "-ite", add the suffix __
"-ous"
What is the electronegativity difference for ionic bonds?
>2.0
What is the lewis dot structure of N2
Al(CN)3
Aluminum Cyanide Ionic
Formula for Ammonium Chloride
NH4Cl
What is the name of H2SO4?
Sulphuric Acid