The students pick two elements from the periodic table which belongs to different groups. The student decided to choose barium (Ba) and phosphorus (P). Determine their charges:
Ba: ____ charge
P: ____ charge
Barium: +2 charge
Phosphorus: -3 charge
The following diagram shows the transfer of electrons to form what ionic compound? (Please provide the chemical name and formula)
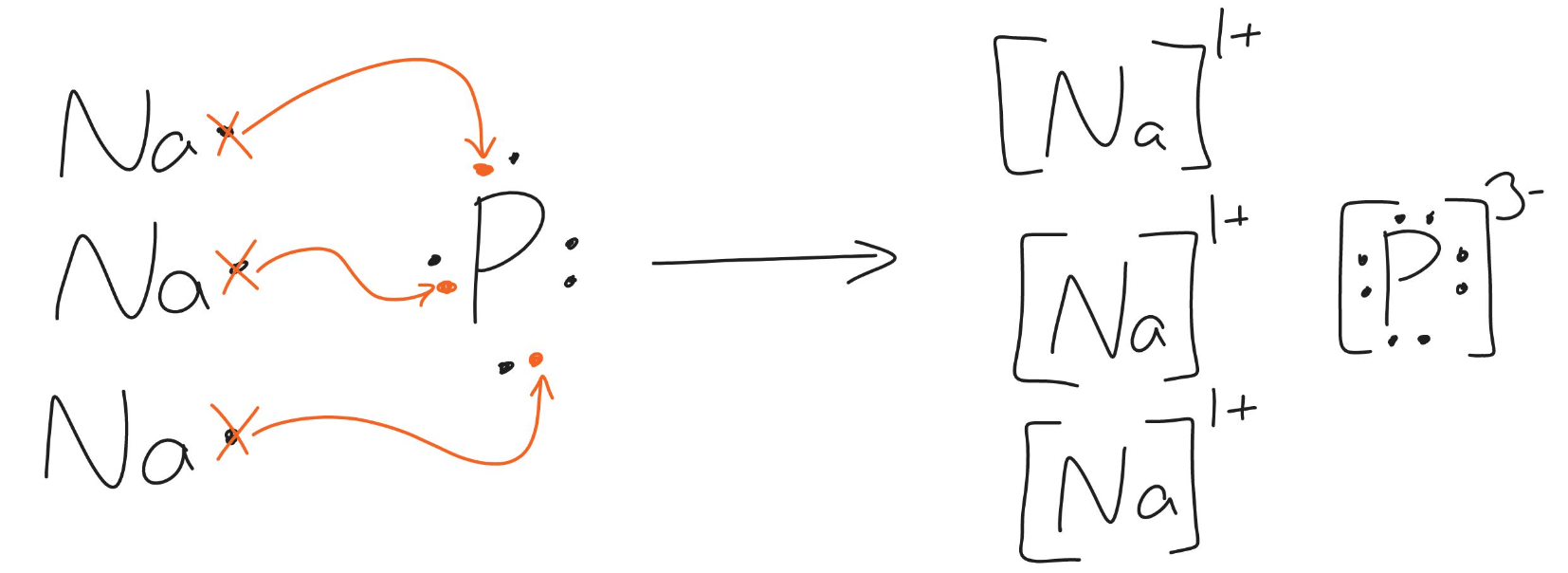
Na3P
sodium phosphide
Name this compound:
N2O4
Dinitrogen Tetroxide
Determine the VSEPR geometry of the center atom circled in red:
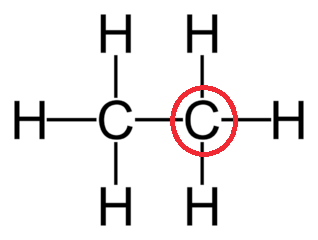
Tetrahedral
1. In this picture, determine the type of attraction the dash lines indicate:
2. Determine if this is considered intermolecular/intramolecular force:
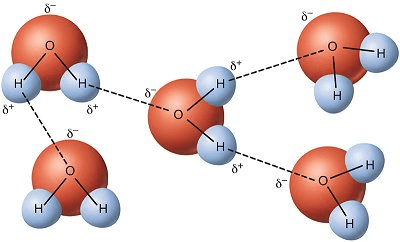
1. Hydrogen Bonds
2. Intermolecular Force (between molecules)
If two elements are in the same group (column), what do they typically have in common?
a) # of valence electrons
b) # of protons
c) charge
a) and c)
Determine the formula and name of the ionic compound that is based on the given Lewis Dot Structures:
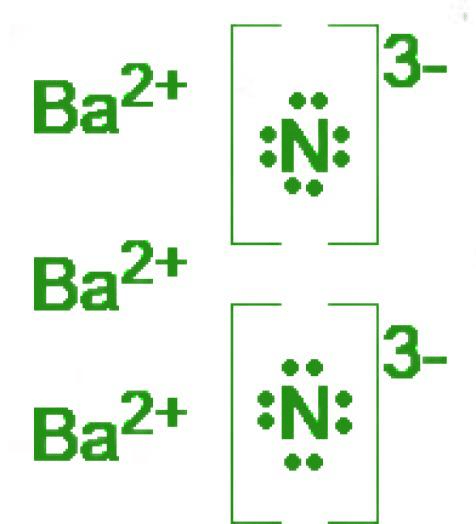
Ba3N2
barium nitride
Name this compound:
NO
Nitrogen Monoxide
Determine the geometry of the center atom circled in green:
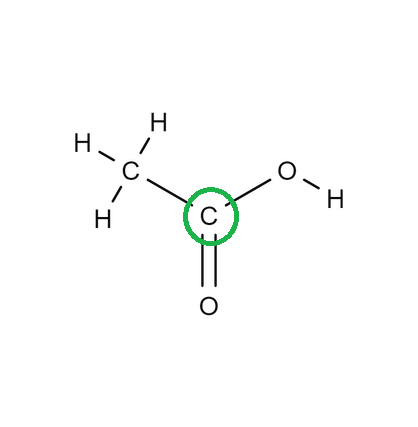
Trigonal Planar
List 3 properties of water, and determine the main reason why water demonstrates these properties.
1. High Surface Tension
2. Low Vapor Pressure
3. Ice (solid) is less than dense than water (liquid)
Primary Reason: Hydrogen Bonding
Fill in the correct words for the following statement:
When forming ions, metals tend to _____ electrons, while nonmetals tend to _____ electrons.
1. lose
2. gain
Draw the Lewis dots structure for CaCl2
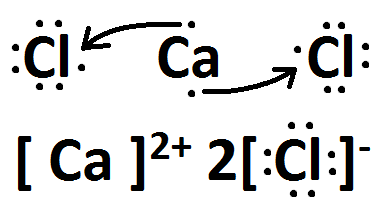
Determine the number of valence electrons and VSEPR geometry of PO33-:
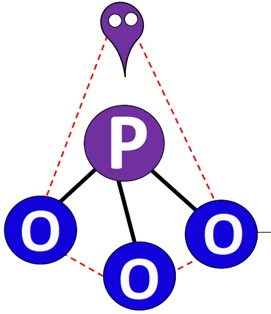
26 valence electrons
trigonal pyramidal
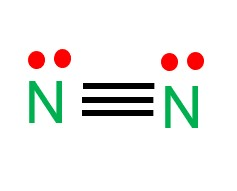
Draw the Lewis structure for a nitrogen molecule (N2) and determine its shape
Linear
Which of the following molecules have the same empirical formula:
a) CCl4 and C2Cl6
b) CH and C6H6
c) C2O6H5 and C4O8H12
d) H2O and H6O3
b) CH and C6H6
d) H2O and H6O3
When forming ions, Calcium loses two electrons while Chlorine atoms gain 1 each in order to become "stable." What is the rule that demonstrates this idea?
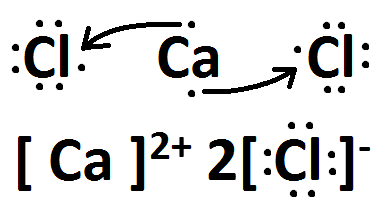
Octet Rule
1. Use the criss-cross reduce method to write the chemical formula for cobalt (II) phosphate.
2. Determine the overall charge of the compound:
1. Co3(PO4)2
2.Net Charge: 0
The difference between a covalent and ionic compound is that electrons are transferred between a(n) _________ and a nonmetal in an ionic bond, whereas electrons are shared between 2 _________ in a covalent bond.
2) nonmetal
Which of the following molecules has a trigonal pyramidal VSEPR geometry?
a) CO32- b) NH3
c) BrF3 d) CO2
b) and c)
Calculate the percent composition of nitrogen dioxide (NO2):
% of Nitrogen: _______
% of Oxygen: ________
% of Nitrogen: 30.45%
% of Oxygen: 69.55%
Determine which elements violate the Octet Rule? Why?
Hydrogen and Helium
These two elements only needs two electrons to become "stable."
Draw the Lewis dots structure for MgF2
This is an ionic compound, therefore, you will need to include brackets on the drawing.
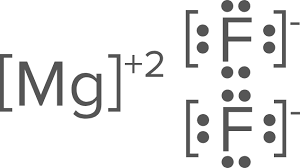
2. Identify which one of the following molecules requires more energy to break apart?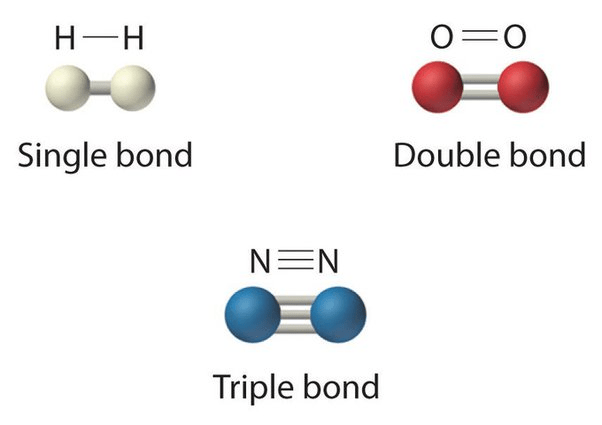
N2 because it contains a triple bond.
Convert 26.0 g of BF3 to moles.
molar mass BF3 = 10.81+3(19.00)=67.81 g/(mol)
26gBF3* (1mol BF3)/(67.81g BF3)=
0.383 mol BF3
Calculate the empirical formula of a compound that is 27.3% C and 72.7% O.
*start by converting the % to g, and g to moles
*divide by the smallest amount of moles after
C: 27.3g*(1mol)/(12.01g)=2.27
O: 72.7g*(1mol)/(16.00g)=4.54
C: 2.27/2.27=1; O: 4.54/2.27=2
empirical formula = CO2