What is the percent composition of Cu in CuSO4?
63.55/159.65 x 100 = 40%
4 NH3 + 5 O2 ----> 4 NO + 6 H2O
If 1 mole of NH3 is added to an unlimited supply of oxygen, how many moles of nitrogen monoxide, NO, will be produced?
1 mole of NO
Balance this equation:
___Cr + ____ O2 ------> ____ Cr2O3
__4_Cr + __3__ O2 ------> __2__ Cr2O3
You have a mixture of the following isotopes with the following percentages and masses. What is the average atomic mass?
75.5 % Cl-35
24.5% Cl-37
35.49 amu
Name the 7 diatomic elements
HONClBrIF
What is the empirical formula of a substance containing 3% H, 32% P, and 65% O?
H3PO4
How many grams of iron (III) oxide, Fe2O3, will be produced from the following reaction if you start with 42.84 grams of iron, Fe?
___Fe + ___ H2O ------> ___Fe2O3 + ___ H2
61.25 g Fe2O3
Balance this equation:
_ Al2(SO4)3 + __ Zn(NO3)2----> _Al(NO3)3 + _ZnSO4
Al2(SO4)3 + 3 Zn(NO3)2----> 2 Al(NO3)3 + 3ZnSO4
The average atomic mass of carbon is 12.01 amu. Carbon has 3 isotopes. C-12, C-13, and C-14. Which of the three isotopes is most common in nature? Explain.
C-12 because the average atomic mass is a weighted average based on percent abundance. The most common isotope's mass is closest to the average mass.
Determine the number of protons, neutrons, and electrons in each of the following particles.
S-2
Al-28
Al+3
S-2: p= 16 n= 16 e=18
Al: p=13 n=15 e=13
Al+3: p=13 n=14 e=10
The empirical formula of a compound is CH. If the molar mass of this compound is about 78 g, what is its molecular formula?
C6H6
How many molecules of sodium chloride, NaCl, will be produced when 5 atoms of sodium, Na, reacts with an excess of chlorine gas?
2 Na + Cl2 -------> 2 NaCl
5 molecules of NaCl
Which contains the most atoms of copper?
1 mole of Cu
1 mole of Cu2O
1 molecule of Cu2O
100 grams of Cu
1 mole of Cu2O
What are isotopes? Give examples.
Atoms of the same element that differ by their mass or number of neutrons.
C-12 vs. C-14
H-1 vs. H-2 vs. H-3
Name the scientist responsible for each of the following developments.
- Atomic theory
- Discovery of electrons
- Discovery of the nucleus of the atom
Atomic theory: John Dalton
Electrons: J.J Thompson
Nucleus: Ernest Rutherford
Tin(II)fluoride is often added to toothpaste as an ingredient to prevent tooth decay. What is the mass of fluorine in 24.6 g of this compound?
5.97 g
6.0 grams of hydrogen gas, H2, are reacted with 30.0 grams of oxygen gas, O2, to produce water.
1. Which reagent is in excess?
2. How many grams of H2O will be produced?
2 H2 + O2 ------> 2 H2O
H2 is the excess reagent
33.75 g of water will be produced
What substance is the limiting reagent in this equation if 1 mole of Na is reacted with 1 mole of F2?
2 Na + F2 -------> 2 NaF
Na - you need 2 moles of Na for every 1 mole of F2
You start with 5 grams of CH4 and burn it in the presence of unlimited O2 producing 6 grams of water. What is your percent yield?
CH4 + 2 O2 -------> CO2 + 2 H2O
Theoretical yield = 11.25 g (stoichiometry)
% yield = 5/11.25 = 44%
How many molecules are in 35 g of sodium sulfate?
Molar mass = 142.05 g
1.48 x 1023 molecules
The two compounds listed below are fertilizers.
1. Which of these is the richest source of nitrogen?
2. What % of N does it contain?
ammonium nitrate: NH4NO3
Urea: (NH2)2CO
Urea - it contains 47% nitrogen. Ammonium nitrate only contains 35%
You react 100 grams of AlCl3 with 50 grams of Ca and produce 100 g of CaCl2. If your percent yield is 80.1%, what is the theoretical yield of CaCl2?
___Ca + ___AlCl3 -------> __CaCl2 + __ Al
124.8 g of CaCl2
What are three quantities that 1 mole equals?
1 mole = molar mass of a compound or element
1 mole = 6.022 x 1023 atoms or molecules
1 mole = 22.4 L
According to pre-lab calculations, a student’s experiment should have produced 2 g of NaCl. When he weighed his product from lab, only 1.5 g was present.
Determine the following from the data
Theoretical yield?
Actual yield?
% yield?
% error?
Theoretical yield = 2 g
Actual yield = 1.5 g
% yield = 75%
% error = 25%
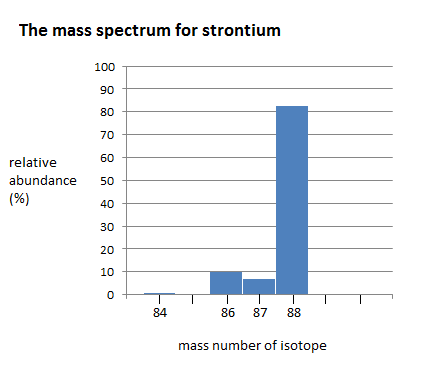
What is the approximate average atomic mass of this element? Describe how you calculated this.
88(.82) + 87(.06) + 86(.10) + 84(.02) =87.66 amu