John Dalton
What unit do we measure the mass of subatomic particles, and what are their masses?
atomic mass unit (amu)
P = 1 amu
N = 1 amu
e = 0 amu
What atomic model do we use to show electron configurations?
Bohr Model
What shell in an atom with three shells has the most energy?
Is it close to or far from the nucleus?
Shell 3, the farthest from the nucleus
What is an ion?
When an atom gains or loses an electrons, it forms an ion.
Protons do not equal electrons
Who did it: Discovered of electrons with a cathode tube ray and developed the plum pudding model
JJ Thompson
Why do chemists use the Bohr model to show electron locations even though we have since disproven the theory?
Simplicity
Ca: 2-8-8-2
(A) How many shells does calcium have?
(B) How many electrons are in the valance shell?
(A) 4 Shells (4 numbers)
(B) 2 electrons (last shell)
What has to happen to excite an electron?
Energy must be absorbed
do NOT say anything about light!
How do we determine the charge of an ion?
Add the positive and negative charges.
Example: 10 P & 11 e
+10 + - 11 = -1
note electrons are added as negative values
Who said it: Electrons circle the nucleus of an atom in fixed orbits
Niels Bohr

Two samples are given:
Sample 1: 10 p, 11 e, 10 n
Sample 2: 10 p, 10 e, 10 n
Are samples 1 & 2 Isotopes
No. Sample 1 is an Ion, sample 2 is an Isotope
Draw a lewis dot diagram for Fluorine

2-7; only valance electrons shown
Explain how an excited electron moving back to it's ground state produces light
As an excited electron moves from a higher energy shell (excited) to a lower one (ground), it releases energy in the form of light. This is what we see!
Draw a lewis dot diagram for fluoride (the ion of fluorine)
Atom: 2-7 ION: Gains electron, 2-8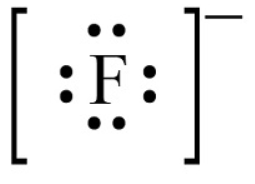
What's the difference between the Bohr and Modern theory?
Bohr: Electrons travel in fixed orbits around the nucleus
Modern: Electrons exist in a probable location around the nucleus called orbitals
An atom of potassium (K) containing 21 Neutrons has a mass number of ______
Atomic Number K: 19
Neutrons: 21
Mass Number = P+N
19+21 = 40
For calcium, write:
(A) Ground State Electron Configuration
(B) Excited State Electron Configuration
Ground: 2-8-8-2
Excited: 2-8-7-3
Name the saying ranking colors from low energy to high energy
ROYGBIV
"red, orange, yellow, green, blue, indigo, violet"
Draw the lewis dot diagram for barium
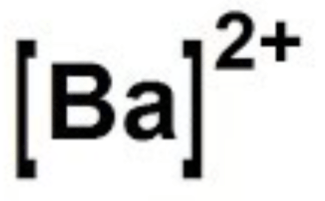
Atom: 2-8-18-18-8-2
Ion: 2-8-18-18-8-0
Show the shell that lost or gained electrons. Since we lost electrons, now we dont see them in our lewis dot diagram
How did Rutherford conclude that the atom is mostly empty space
DOUBLE JEAPORDY: ALSO Include how he concluded the nucleus is a hard, dense, positively charged core
(Choosing group up to triple points)
Rutherford concluded that the atom is mostly empty space because most alpha particles went straight through gold foil in his famous experiment.
He concluded the nucleus is dense because some alpha particles bounced to the side (did not go straight through the foil).
What is the nuclear charge of a potassium ion?
Potassium has 19 Protons, so +19
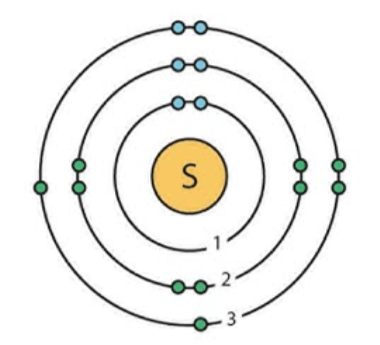
Draw the bohr model of sulfur
2-8-6
Relate wavelength and energy
Longer wavelength = LOWER energy
Shorter wavelength = HIGHER energy
DOUBLE JEOPARDY
Which of the 4 samples have the same number of electrons?
Ca+2, K+, S-2, Si+2
We will go over on board
Ca+2, K+, S-2 all have 18 electrons