2.86 g
2H2 + O2 --> 2H2O
If 0.500 mol of H2 reacts with 0.300 mol of O2, which reactant is limiting?
H2
Name all 7 strong acids.
HCl, HBr, HI, HNO3, H2SO4, HClO4, HClO3
A sample of hard water is to be analyzed for the presence of Mg+2 ions. Suggest an aqueous solution that could be added to the water to precipitate the magnesium.
What technique separates mixtures based on differences in solubility at various temperatures?
Recrystallization
How many moles of nitrate ions are present in 2.36 mol of calcium nitrate?
4.72 mol
Mg + 2HCl --> MgCl2 + H2
If 2.0 g of Mg is added to 0.30 mol of HCl, what volume of H2 gas can be produced?
1.87 L
An aqueous solution contains Li+, NH4+, and Ca+2 ions. A solution of sodium carbonate is added to the beaker, and a precipitate forms. What is the identify of the precipitate?
CaCO3
What is the purpose of gravimetric analysis?
To determine the amount of analyte in solution by precipitating it, and weighing the precipitate.
What element is shown by the mass spectrum below?
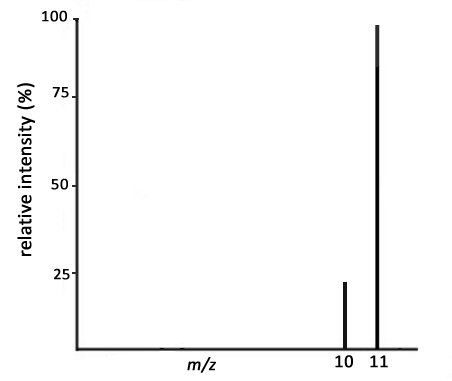
Boron
A hydrocarbon undergoes combustion analysis, and produces twice as many moles of water as it does carbon dioxide. What is the empirical formula of the hydrocarbon?
CH4
CaCl2 + 2AgNO3 --> 2AgCl + Ca(NO3)2
Equal moles of CaCl2 and AgNO3 are reacted in aqueous solution. Draw a particulate diagram to show what species are present in the vessel after the reaction is complete.
Drawing should contain:
- AgCl(s)
- Dissociated calcium nitrate
- Excess dissociated calcium chloride
Write the balanced, net ionic equation for the following reaction:
An aqueous solution of silver nitrate is added to a solution of lithium carbonate.
2Ag+ + CO3-2 --> Ag2CO3(s)
The calculated mass would be too low. Adding too much water could dissolve some of the precipitate, so it would appear that there is less Mg in the sample.
How should a student record the volume reading below?
Ex: 25.8 mL
A compound with a molar mass of 34.00 g/mol is found to consist of 0.44 g H and 6.92 g O. Find its molecular formula.
H2O2
NO CALCULATOR.
When 68.0 g of hydrogen peroxide decomposes, what mass of each product is created?
2H2O2 --> 2H2O + O2
(68.0 g of hydrogen peroxide = 2 mol)
= 36 g H2O and 32 g O2
Write out the balanced, net ionic equation for the following:
Magnesium metal is added to aqueous hydrochloric acid, and bubbles form.
Mg(s) + 2H+ --> Mg+2 + H2(g)
A piece of jewelry is analyzed for the presence of Ag+ ions. The jewelry is dissolved in nitric acid, and aqueous sodium carbonate is added to precipitate the silver. If the mass of Ag2CO3(s) collected is 2.00 g, what mass of Ag+ was present in the sample?
1.56 g
What is the correct chemical formula for pentanol?
C5H11OH
A sample of ethanol, C2H5OH, is analyzed using combustion analysis, and is found to contain a higher percentage by mass of hydrogen than the accepted value.
The chemist hypothesizes that if the sample is contaminated with C6H6, this could account for the higher %H. Is this hypothesis valid? Explain.
No. Ethanol is 13% by mass of hydrogen, and the impurity is only 8% by mass of hydrogen. Therefore, this impurity would lower the calculated percentage of hydrogen in the sample.
NO CALCULATOR!
When 3 mol of sodium metal reacts with 3 mol of bromine gas, 2 mol of sodium bromide is produced. What is the percent yield of sodium bromide?
66%
When a certain reactant is added to aqueous acetic acid, bubbles begin to form.
Is the reactant most likely to be sodium chloride, sodium carbonate, or sodium phosphate? Explain by writing a balanced, net ionic equation.
Sodium carbonate:
2HC2H3O2 + CO3-2 --> 2C2H3O2- + CO2 + H2O
CO2(g) is the bubbling!
A metal phosphate, M3PO4, is studied using gravimetric analysis. 5.00 g of the metal phosphate is reacted with calcium nitrate, forming 6.68 g of precipitate:
2M3PO4 + 3Ca(NO3)2 --> 6MNO3 + Ca3(PO4)2
Based on this information, determine the molar mass, and the identity of the metal phosphate.
Li3PO4 (MM= 116 g/mol)
What is the correct chemical formula for sodium thiosulfate?
Na2S2O3