The presence of this separation of charge is present in polar molecules and creates an intermolecular force of medium strength.
What is a dipole?

This force is largely responsible for molecule Z having a greater boiling point than molecule Q
What are dipole-dipole attractions?
This is the number of moles of a gas that takes up 5.0 L at 4.0 atm and 200. K.
What is 1.2 moles?
n = PV/RT = (5*4)/(200*273)

At equal temperatures, this sample will have a higher pressure inside the container.
(Must explain why)
What is sample B, due to more particles/moles of gas present?

This is the hybridization of the C atom in molecule Q indicated by the arrow.
What is sp hybridization?
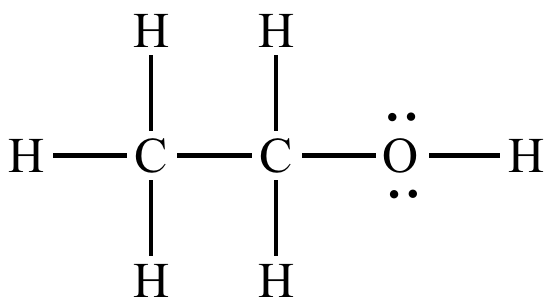
This is the strongest intermolecular force that this particular substance can form.
What is hydrogen-bonding?
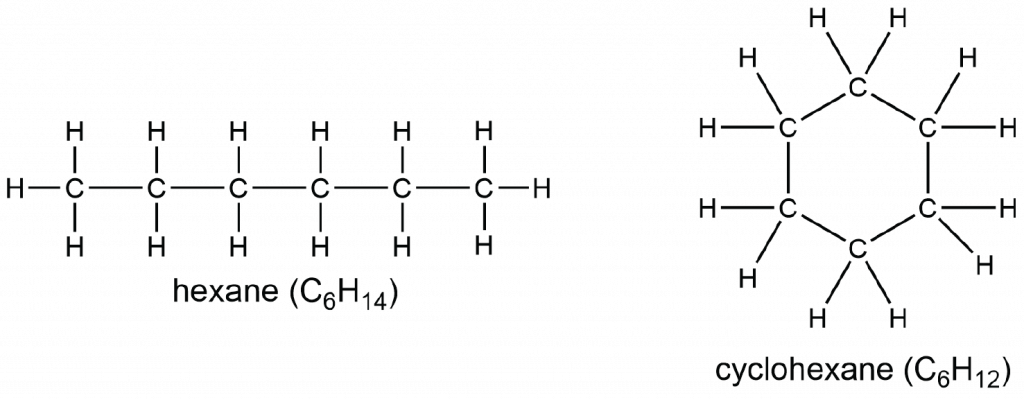
Between these two substances, this substance will exhibit greater London Dispersion Forces
What is hexane?

In a laboratory where this experiment was performed, the temperature is 295 K and the pressure in the gas-collecting tube is 744.7 mmHg. If the vapor pressure of water at 295 K is 19.8 mmHg, determine the pressure of the gas in the tube.
What is 724.9 mmHg?
Pgas = 744.7 - 19.8
 The Maxwell-Boltzmann curves above represent equimolar samples of the same substance. This sample has the lowest temperature.
The Maxwell-Boltzmann curves above represent equimolar samples of the same substance. This sample has the lowest temperature.
What is sample A?
(peak is furthest left)

Between the C-H bonds in Q and the S-H bond in Z, this bond will be shorter.
(Double points for explaining why)
(full points for explanation)
What is the C-H bond?
From the following list, this substance will have the weakest dispersion forces.
H2, O2, Xe, F2
What is H2 ?
These elements are present in covalent network solids?
(There are 3 answers I will accept, 100 pts for each).
What are B, C, and Si?
This is the total pressure in a 1 L tank after adding a 2 L sample of N2 and a 1 L sample of Ar, each originally at 1 atm and 273 K.
What is 3 atm?
N2 new pressure: (2*1) = PN2 = 2
Ar new pressure: (1*1) = PAr = 1
PT = PN2 + PAr= 2+1 = 3
A sample of gas is originally at held at 290 K and 2.4 atm in a 1.5 L piston. The piston expands to 3.0 L, causing the pressure to change to 1.2 atm. This is how the average kinetic energy of the gas particles changes.
What is stays the same?
(no change in T)
This is the electron configuration of the Sc2+ ion.
What is 1s2 2s2 2p6 3s2 3p6 3d1

These are all the intermolecular forces present in molecule Q and in molecule Z.
You must correctly identify all the intermolecular forces present in both molecules for full points.What are LDFs in Molecule Q and LDFs and dipole-dipole attractions in Molecule Z?

Of the three substances pictured, this one will have the highest boiling point.
(For full points, you must correctly explain why)
A flask that contains 0.25 mole of SO2(g), 0.50 mole of CH4(g), and 0.50 mole of O2(g). The total pressure of the gases in the flask is 800 mm Hg. This is the partial pressure of SO2(g)
What is 160 mmHg
PSO2 = (mole fraction of SO2) * PT
Mole fraction = 0.25/(0.25 + .5 + .5) = 0.2
PSO2 = 0.2 * 800
This is a particle-level explanation for why the pressure of a gas increases as it is heated up.
As gas molecules move faster, there are more collisions with the container it is in.
This is the molecular geometry of XeF4, pictured below.

What is square planar?

These types of interactions will form when benzene, pictured above, comes in contact with water, H2O.
Answer comes from the following:
hydrogen bond , London Dispersion Forces , dipole-dipole attraction , dipole-induced dipole
What are LDFs and dipole-induced dipole?

Molecule D has a greater melting point than molecule E. This difference between the two molecules explains why.
What is a greater polarizability (due to more electrons) or greater polarity (due to greater electronegativity difference between Se-F and Se-O compared to S-F and S-O)?
This is the molar mass of a 4.0 g sample of gas that fills a 2.0 L container at 0°C and 1 atm.
What is 44 g/mol?
MM = g / mol = 4 / (mol gas)
mol gas (n) = 2*1/(273*0.08206)
OR
MM = DRT / P = (4/2)(0.08206)(273)/1
A student cools a sample of O2(g) to -180°C (the boiling point of O2(l) is -183°C). This is how the actual volume of the gas will compare to the volume calculated using the ideal gas law.
(double points if you can explain why)
What is lower than calculated?
(As gas starts to slow down, it lacks the energy to overcome IMFs and starts to condense, creating less average space between particles)
This is the number of hydrogen atoms in 4.3 grams of hexane, C6H14.
What is 4.2 x 1023 atoms H?
4.3*(1 mol/86 g)*(14 mol H/1 mol C6H14)*(6.02 x 1023 atoms / 1 mol)