Shredding paper is an example of __________
What is a physical change?
What is the definition of density?
What is the amount of mass in a given volume?
This type of reaction feels cold.
What is endothermic?
This type of bond happens when two elements share electrons.
What is covalent bond?
These are all descriptions of what:
- Pure Substance
- Cannot be broken down into other substances
What is an element?
Melting ice cubes is an example of _______
What is the density of an object with a mass of 40g and a volume of 8 mL?
What is 5 g/mL?
Water freezing is this kind of reaction.
What is exothermic?
An example of what kind of bond is NaCl.
What is ionic bond?
These are descriptions of what:
- Made up of one or more substances
- Still considered a pure substance
What is a compound?
Density is a _______ property
What is physical?
This is how you calculate the volume of an irregular object.
What is taking the initial volume and subtracting it from the final volume?
This type of reaction feels hot on the outside.
What is exothermic?
This type of bond donates/accepts electrons within two elements.
What is an ionic bond?
This can be classified as homogenous and heterogenous. Made up of pure substances and compounds.
What is a mixture?
Oxidation is an example of a _______ ________
What is a chemical change?
The density of the following object is _______ g/mL.
Mass= 45g
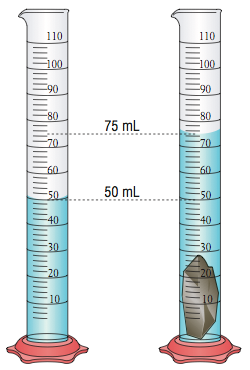
What is 3?
Baking soda and vinegar is this type of reaction.
What is endothermic?
Water is an example of what kind of bond.
What is Covalent?
Coffee is an example of what kind of mixture.
Trail Mix is an example of what kind of mixture.
What is homogenous and heterogenous?
Is it a physical property or chemical property? What is it?

What is a chemical property? What is oxidation?
Which of the following liquids was most dense in our lab? least dense?

what is Salt water and Alcohol?
An ice cube melting on a counter is what kind of reaction.
What is endothermic?
This type of bond is the strongest kind.
What is covalent bond?
Element, Compound, or Mixture?
What is a compound?