Draw the Lewis electron dot diagram for chlorine (Cl).

Use the criss-cross method to write the chemical formula for sodium chloride. Show your work!
NaCl
What should the charges of the cation(s) and anion(s) in an ionic compound add up to equal?
0
What is the name of the anion that phosphorus forms?
phosphide
Draw the Lewis dot diagram for aluminum (Al).

Use the criss-cross method to write the chemical formula for barium iodide. Show your work!
BaI2
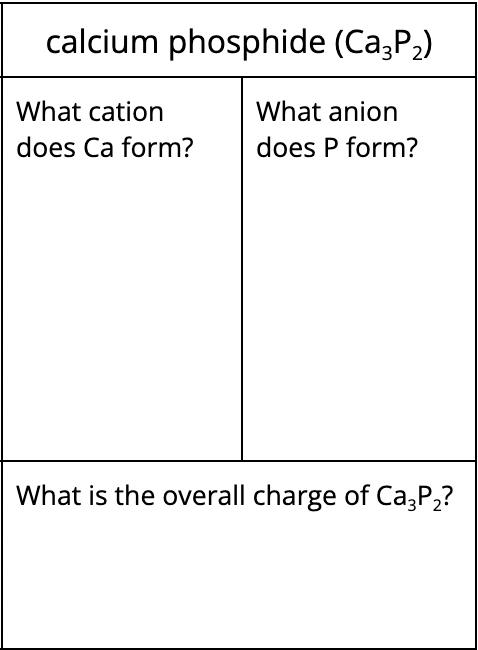
What is the chemical name for RbP3?
rubidium phosphide
Use Lewis electron dot diagrams to show the formation of rubidium fluoride.
Rb + F --> Rb1+ [F]1-
What is the chemical formula for the ionic compound formed from Be2+ and C4- ions?
Be4C2
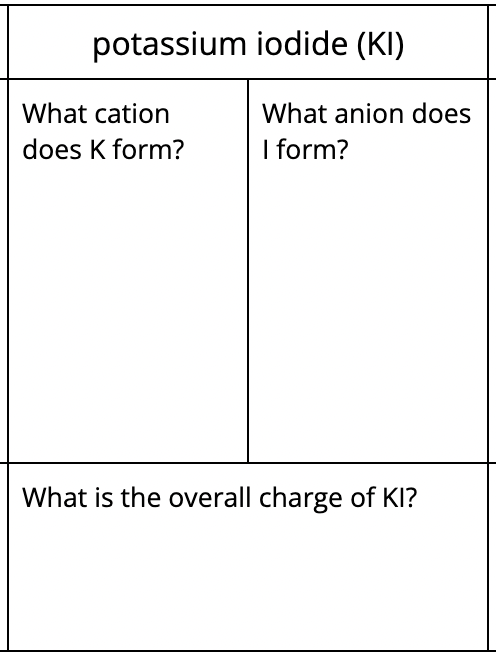
Write the name for KBr.
potassium bromide
Draw the formation of radium sulfide using Lewis dot diagrams.
Ra + S --> Ra2+ [S]2-
Write the chemical formula for magnesium telluride.
Mg2Te2
What is the name of the anion that fluorine forms?
Draw the formation of AlBr3 using Lewis dot diagrams.
Write the chemical formula for aluminum oxide.
Al2O3
What does it mean when we say that ionic compounds are “electrically neutral”?
Name the following ionic compound: GaN
gallium nitride