In the Chemical formula MgBr2 what does the 2 stand for?
The number of __________ in the atom of an element determines its characteristics
Valence electrons
Looking at the Periodic Table tell me 3 elements of the most non-reactive metals 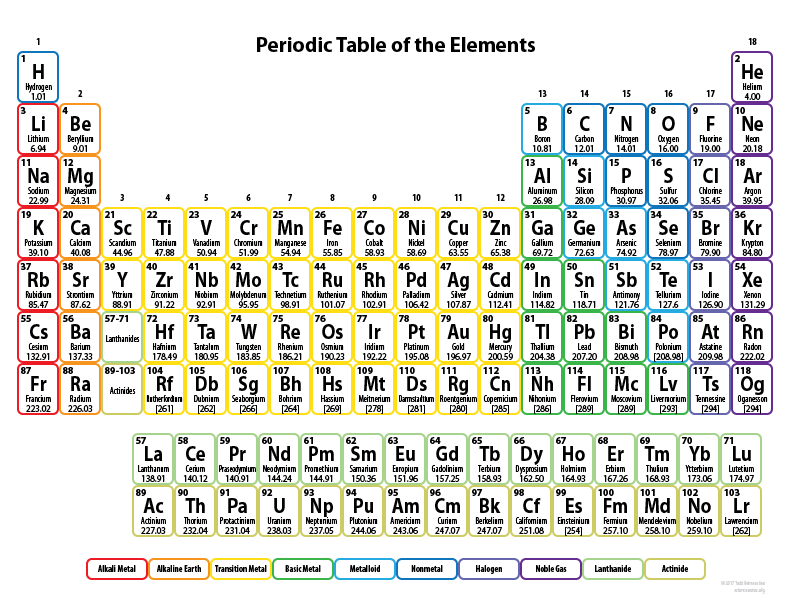
F, Cl, Br, I, At, or Ts
How many dots would you put around Gallium?
3
Atoms tend to be stable and nonreactive when they have how many electrons?
8
Two pairs of electrons shared between two atoms are called a what?
The attractive force that hold two atoms together is called ______
chemical bond
What is the difference between group and period?
Groups are the columns and Periods are the rows. Groups tend to have like chemical characteristics while periods have the same energy levels.
If there are 2 dots around Ca what group does it belong too?
2
Ionic bonds form between two ions that have ____?
opposite charges
Opposites attract
When an atom gains an electron it becomes a _____ion?
negative
Within each _______ in the periodic table, elements have similar properties because they have the same number of valence electrons
group
Which group or groups contain the most reactive elements?
Group 1 or the Alkali Metals
Oxygen is in group 16 it has how many dots? How many more are needed to make it full?
6 dots and needs 2 more
In order to have a stable arrangement of 8 valence electrons, metal atoms are likely to _________ electrons.
lose
Ionic compounds that dissolve in water conduct electricity because they break into _______________ that move freely.
When naming ionic compounds which is written first?
How many protons does Sn (Tin) have?
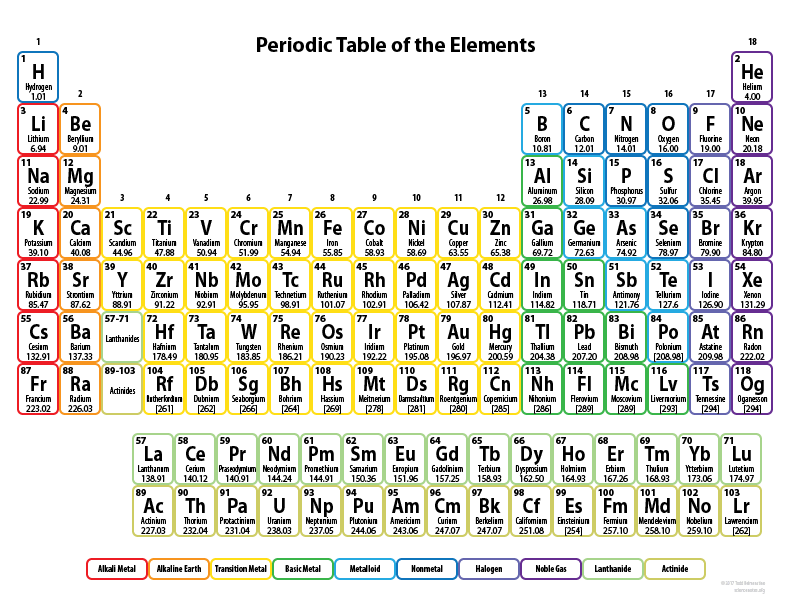
50
Can you add Radon and Francium together why or why not?
No because Radon is a Noble Gas and its Valence electron shell is already full adding one more to the shell would put it over the magic number of 8.
What is the definition for Triple Bond?
A chemical bond formed when atoms share three pairs of electrons.
A metal crystal consists of positively charged metal ions embedded in a "sea" of freely moving
Valence electrons
How would you name the chemical Na2S?
Sodium sulfide
Gallium belongs to group 13 and has 3 valence electrons. How many valence electrons does Polonium have?
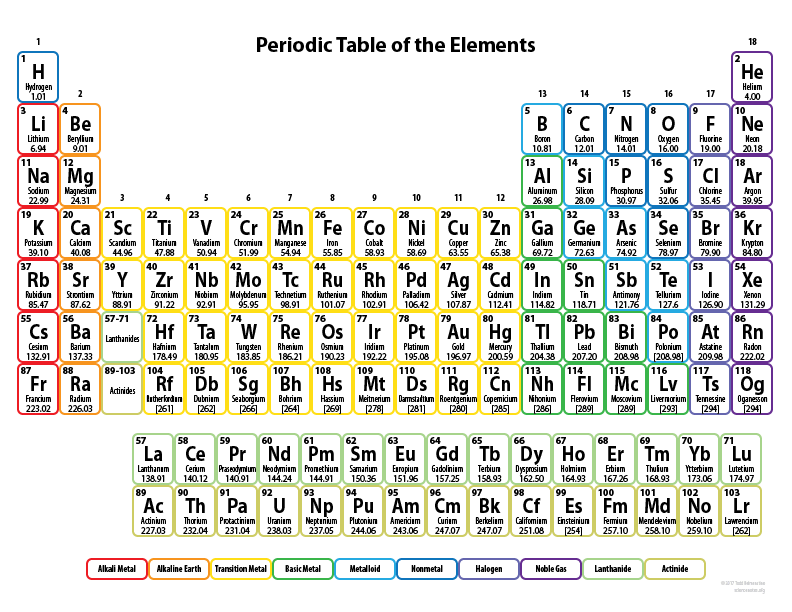
6
What element could I add to group 15 to make it stable?
Anything from group 3
or one element from group 2 and one from group 1
or I could add 3 from group 1.
Magic number is 8!
What is the definition for molecule?
A neutral group of two or more atoms held together by covalent bonds.