The force of attraction between nonpolar molecules:
London dispersion forces
A material's ability to conduct heat or electricity is classified as this type of property
Physical property, Qualitative, Intensive
A student constructs composed of oil (density of oil = 0.90 g/mL), water (density of water = 1.0 g/mL), and alcohol (density of alcohol = 0.79 g/mL). Due to density, the three liquids separate in this order
Water at the bottom
Oil in the middle
Alcohol at the top
A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, the density of copper is calculated to be
8.92 g/cm3
Which of the following tests will MOST help them to infer the strength of intermolecular forces between ionic and covalent compounds? 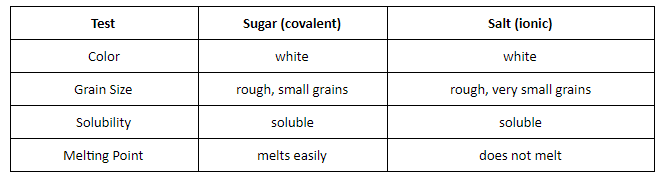
Melting point
The strength of temporary dipoles _______ with the size of the molecule
The ability for a metal like zinc to react with an acid like hydrochloric acid is classified as this type of property
Chemical property, Reactive
Two students are conducting an experiment with unknown Liquid A and unknown Liquid B. The students drop a penny into each test tube and measure how fast the penny falls to the bottom of the test tube. It takes the penny 10 s to fall through Liquid A and 5 seconds to fall through Liquid B. From this information, the students decide that this liquid with more viscous.
Liquid A
A student measured out 35.4 mL of sulfuric acid, which has a mass of 65.14 g. The student then calculates the density of sulfuric acid to be
1.84 g/mL
Based on the data, which substance has the strongest intermolecular forces? 
Substance 2
What type of intermolecular forces is observed in the following substance:
H2O
Hydrogen Bonding
This type of property describes values such as weight, mass, and volume
Quantitative property or extensive property
When performing the Coke experiment, students discovered that Diet Coke floats but regular Coke sinks. This is because
Diet Coke is less dense than regular Coke
(Bonus points if students include that the reason diet coke is less dense is because it doesn't have any sugar whereas regular coke does have sugar)
An irregularly-shaped sample of aluminum (Al) is placed on a balance and found to have a mass of 43.6 g. The student decides to use the water-displacement method to find the volume. The initial volume is 25.5 mL and after the Al sample is added, the water level has risen to 41.7 mL. The student discovers that the density of aluminum is
2.69 g/mL
A student places two substances into a Bunsen burner. Substance A melts and then ignites. Substance B does not change. Which substance is has the strongest intermolecular forces and why?
The higher the boiling point, the stronger the intermolecular forces. Since substance B does not change, it will have the stronger intermolecular forces.
What type of intermolecular forces is observed in the following substance:
HCl
Dipole-Dipole
This type of property does not describe the amount of matter present but describe quality likes luster and texture
Intensive property or qualitative property
A student constructs composed of oil (density of oil = 0.90 g/mL), water (density of water = 1.0 g/mL), and alcohol (density of alcohol = 0.79 g/mL).The student then drops a bottle cap in the column, which comes to float in between the oil and alcohol layers. The student concludes that the density of the bottle cap is
Between 0.79 g/mL and 0.9 g/mL
The mass of ethyl alcohol (density = 0.789 g/mL that exactly fills a 200.0 mL container is
157.8 g
Arrange the following set of compounds in order of increasing boiling point temperature: HCl, H2O, SiH4
SiH4 < HCl < H2O
LDF < Dipole-Dipole < Hydrogen Bonding
What type of intermolecular forces is observed in the following substance:
CBr4
London Dispersion Forces
pH describes how acidic or basic a substance is:
Chemical, Reactive
The following liquids are poured together in a beaker: alcohol (density=0.79), corn syrup (density=1.38), water (density=1.0), and cooking oil (density=0.93). Which of these liquids will sink to the bottom of the beaker?
corn syrup (density=1.38)
This volume of silver metal (density of silver = 10.5 g/mL) will weigh exactly 2500.0 g.
238.1 mL
Which of the following will have the highest boiling point: HF, HBr, HI, HCl
HF has hydrogen bonding
All of the others are dipole-dipole interactions
Which means HF will have the highest BP because it has the strongest IMFs
If CCl4 and H2O were mixed together, what would be the intermolecular forces between these molecules?
Induced-Dipole Interactions
Mercury is toxin to humans and animals and, the more that is ingested, the more likely it is to cause harm to the individual.
Chemical, Reactive, Extensive
Under what conditions will an object float on water? Be specific.
The object must be less dense (less than 1.0 g/mL) than water
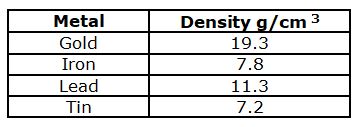
A sample of an unknown substance has a mass of 28.8 grams and a volume of 4 cm3. Based on it’s density, it could be which of the following substances?
Tin
Many covalent substances are volatile liquids or gases at room temperature because
the intermolecular forces of attraction holding the molecules together are weak.