What is outermost or valence?
Made of these charged particles. (Two kinds)
Covalent defined. (What is the definition?)
What is a compound who share at least one pair of valence electrons?
What is electronegativity?
Na4C
Sodium carbide
What does VSEPR stand for?
What is Valence Shell Electron Pair Repulsion?
What is the Lewis dot structure for Barium?
What is *Ba* ? (Two dots on any separate side of the symbol will do.)
Carbonate, Phosphate, Hydroxide, and Ammonium are all examples of these.
Halogens, Chalcogens, and some of the Pnictogens and Crystalogens can covalently bond because of this common characteristic.
What is nonmetals?
What we call a battle over electrons when it is completely transffered.
What is Ionic?
OS
Oxygen monosulfide
What is the name of this shape?
What is tetrahedral?
Each line connecting a pair of elements represents what?
What is two electrons being shared?
Iron (II) oxide can written more simply as what?
What is FeO?
The name of PF5.
What is phosphorous pentafluoride?
The minimum difference in electronegativity in order to be a polar covalent bond.
What is 0.5?
CrCl2
Chromium (II) Chloride
What shape is this?
Bent
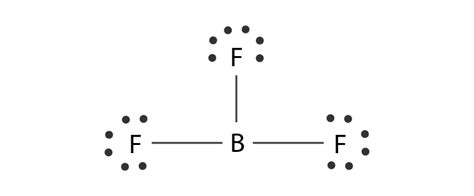
The Lewis Dot structure of BF3
Calcium phosphate's formula.
What is Ca3(PO4)2
Trinitrogen dioxide's formula.
N3O2
The polarity of bond between two hydrogen atoms.
C9F7
What is nonacarbon heptafluoride?
What shape is this?
What is trigonal pyramidal?
What is the Lewis Dot Structure of tetracarbon octahydride?
Four properties of Ionic Compounds.
What is high boiling/melting points
What is brittle and hard
What is conductors of electricity melted or dissociated in solution
What is soluble in water
Four properties of covalent compounds.
What is low boiling/melting points.
What is soft, pliable, waxy, etc.
What is a nonconductor of electricity.
What is usually insoluble in water.
The difference in electronegativity between Lithium and Fluorine.
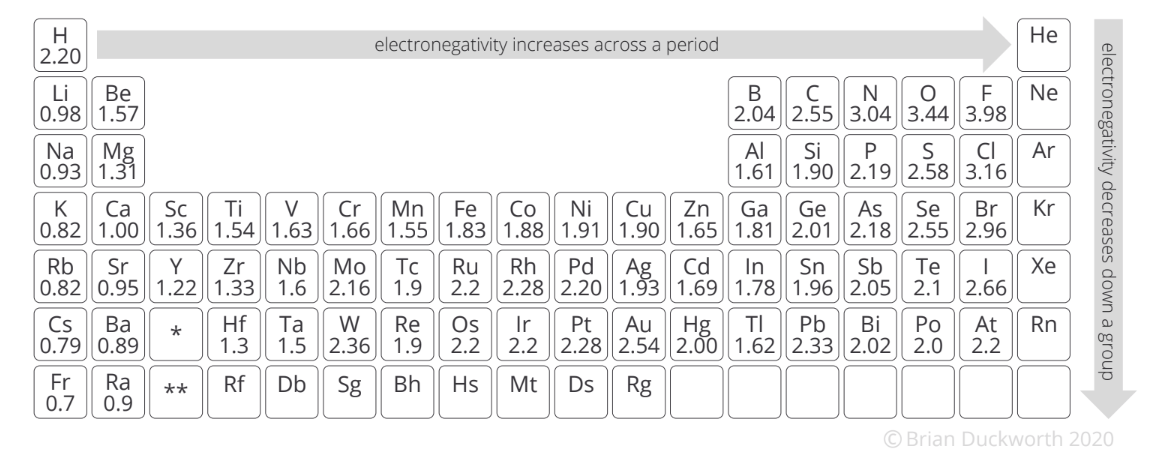
What is 3?
(NH4)3PO4
What is ammonium phosphate?
What is the shape of sulfur difluoride?
What is bent?