What is the definition of an electron?
The part of the atom that is located on the outside of the nucleus, has a negative charge, and is responsible for reactivity (chemical reactions).
What is the definition of a Solid?
The state of matter when atoms are closely packed and touching one another while vibrating.
In the center of the atom.
Describe the particle motion of a solid.
Atoms or particles are packed tightly, close to one another, and vibrating.
According to the diagram, how many phase changes are there?
Six
What is the definition of a proton?
The part of an atom that is located in the nucleus, has a positive charge, and is responsible for determining the type of element of the atom.
What is the definition of a Liquid?
The state of matter in which its atoms are closely packed and touching, but the atoms can move around each other and flow.
What is found in the nucleus of an atom?
Protons and Neutrons
Describe the particle motion of a liquid.
Atoms or Particles are closely packed and touching one another, but can flow freely around each other.
What happens to the energy in the Melting phase change?
What is the definition of charge?
The property of matter to attract or repel.
What is the definition of a Gas?
What part of the atom is responsible for reactivity (chemical reactions)?
The electrons
Describe the particle motion of a gas.
Atoms or particles are spread apart, not touching, and move with high energy to fill the container they are in.
What happens to the energy in the Vaporization phase change?
Energy increases, changing the matter from a liquid into a gas.
What is the smallest unit of matter?
An atom.
What is the definition of a Phase Change?
When matter changes from one state (solid, liquid, gas) to another because of a change in energy.
What subatomic particle does NOT have a charge?
Neutrons
Which state of matter does this sentence describe?
"There is no pattern in the arrangement of particles of water."
A gas
What happens to the energy in the Freezing phase change?
Energy decreases and matter will change from a liquid to a solid.
What is the difference between Energy and Matter?
Matter is measured by mass and volume, but energy is not.
What is the definition of an Element and give three examples of an element?
A unique particle of matter that is a fundamental building block of matter. Examples can come from the periodic table.
How many protons are found in the center of this atom? 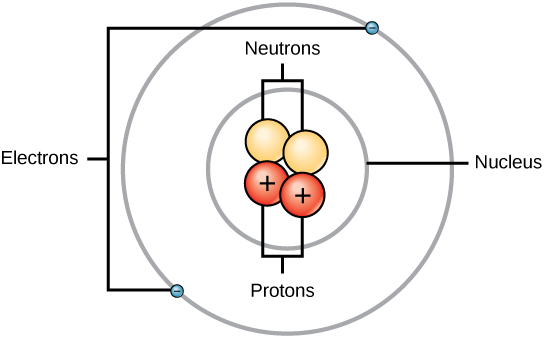
Two protons
Which state of matter does this sentence describe?
"The water has no definite shape."
A liquid.
What phase change happens when Dry Ice (CO2) it dropped into a cup of water?
Sublimation (Solid to a Gas)