The correct SI unit for length
What is meter?
The balance records all measurements 1.5 g too high. This is what type of error?
What is systematic error?
The number of significant figures in 0.00700 g.
These data are accurate, precise, neither, or both:
0.345 g
0.344 g
0.346 g
0.344 g
What is precise?
The density of an object with a mass of 45.6 g and a volume of 26 mL
The correct SI unit for amount of substance
What is mole?
The balance fluctuates with the air currents. This is what type of error?
What is random error?
What is 0.000245 cm?
These data are accurate, precise, neither, or both:
the density of aluminum = 2.71 g/cm3
7.84 g/cm3
7.83 g/cm3
7.86 g/cm3
7.85 g/cm3
What is precise?
The volume of an object with a density of 7.85 g/mL and a mass of 36.5 g.
What is 4.65 mL?
Convert 215 m to km.
What is 0.215 km?
The measurement of this balance.
What is 0.573 g?
The number of significant figures in 1250 g.
What is three significant figures?
Temperature units of Jemicy thermometers.
What is Celsius?
The mass of an object with a density of 2.710 g/mL and a volume of 26.7 mL.
What is 72.4 g?
Convert 1.25 s to ms.
The measurement for this graduated cylinder.
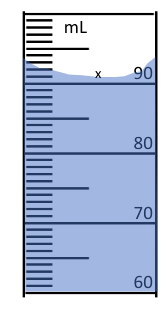
Round 0.005637 mL to two significant figures.
What is 0.0056 mL?
The theoretical lowest temperature that exists.
What is absolute zero?
An object has a mass of 24.6 g and a volume of 2.75 mL. It is which substance:
Water: 1.00 g/mL
Aluminum: 2.71 g/mL
Steel: 7.85 g/mL
Copper: 8.94 g/mL
What is copper?
Convert 0.00640 kg to mg.
What is 6.40 x 103 mg?
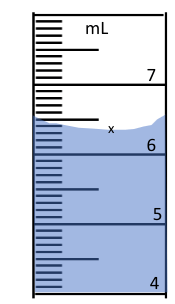 The measurement of this graduated cylinder.
The measurement of this graduated cylinder.
What is 6.32 mL?
Round 35,800 m to four significant figures.
What is 3.580 x 104 m?
These data are accurate, precise, neither, or both:
the density of steel is 7.85 g/cm3
7.84 g/cm3
7.85 g/cm3
7.85 g/cm3
7.86 g/cm3
What is both accurate and precise?
You calculate your density for tin as 6.94 g/mL. The literature value is 7.31 g/mL. Find the percent error.
What is 5.06%?