Sugar dissolving in water is an example of a ____________ change.

Physical Change
Endothermic and Exothermic Reactions are measured by evaluating the ___________ of a sample.
Temperature
In a covalent bond, electrons are ___________ between atoms.
Shared
In a chemical reaction, reactants are found on the ______ side of the "yield" sign.
products = right
reactants = left
Who are the four 8th grade science teachers?
Grupp, Markley, Hartnett, Verne
Why would a balloon inflate when baking soda is combined with vinegar?
Hint: use chemical change or physical change in your answer
A chemical change occurred which caused gas to be released.
If I needed an effective cold pack to treat an ankle sprain, I would want to create an ___________ reaction which causes a __________ in temperature.
Endothermic
Decrease
A type of bond that forms between a metal and a non-metal
Ionic Bond
List ALL of the reactants in the following chemical equation:
Na + MgF2 ---> NaF + Mg
Na
MgF2
Who are the two 8th grade science TAs?
Identify all of the following:
Fireworks exploding is a ________ change
A nail rusting is a __________ change
- chemical
At the beginning of a chemical reaction, the initial temperature of the sample was 35 degrees Celsius. After the reaction was complete, the final temperature of the sample was 90 degrees Celsius.
This is an example of a ________ _________.
Exothermic Reaction
What type of bond will form between the following elements:
Element 1: Mg
Element 2: O
Ionic Bond
Is the following chemical reaction balanced (yes/no)
Prove it by listing out the total # of atoms for each element on the reactants and products sides.
CaCl2 + NaF ---> NaCl + CaF2
No, it is not balanced
Reactants: Ca = 1; Cl = 2; Na = 1; F = 1
Products: Ca = 1; Cl = 1; Na = 1; F = 2
Which Assistant Principal is in charge of Science at Fred Lynn?
Mr. Wood
Provide 2 examples of physical changes that a scientist might observe.
(More than 2 possible answers)
- Phase change
- Change in shape of object
- A solid dissolving in water
In a chemical reaction, when there is a HIGH amount of energy released when new bonds are made the chemical reaction will be _____________.
Exothermic
Describe what is happening in the picture. Include the type of bond and what is occurring to the electron.
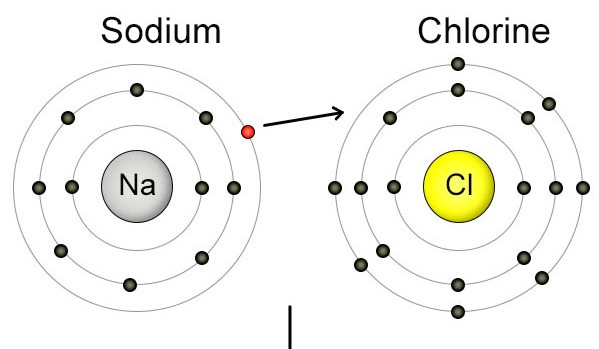
Sodium (metal) is forming an ionic bond with chlorine (non-metal).
The electron is being transferred from the sodium atom to the chlorine atom.
Balance the following chemical equation:
Cl2 + KI ---> KCl + I2
Cl2 + 2KI ---> 2KCl + I2
Which two types of science do we learn in 8th grade?
Physics and Chemistry
What are 3 observations a scientist might note to indicate that a chemical change occurred?
More than 3 possible answers.
Possible answers:
- Bubbles (fizz)
- odor released
- color change
- formation of a new compound
- heat or light released
In an exothermic reaction, the amount of energy required to break the bonds to start the reaction is [less / more] than the amount of energy [absorbed / released].
less, released
A bond between two elements that are poor conductors of electricity would most likely be a __________ bond.
Therefore, the atoms would _________ electrons.
Covalent
Share
Balance the following chemical equation:
Na + HCl ---> H2 + NaCl
2Na + 2HCl ---> H2 + 2NaCl
Describe to me the difference between a scientific theory and a scientific law
A theory explains WHY something happened.
A law explains WHAT happened