Identify each of the following as ionic, metallic, or covalent:
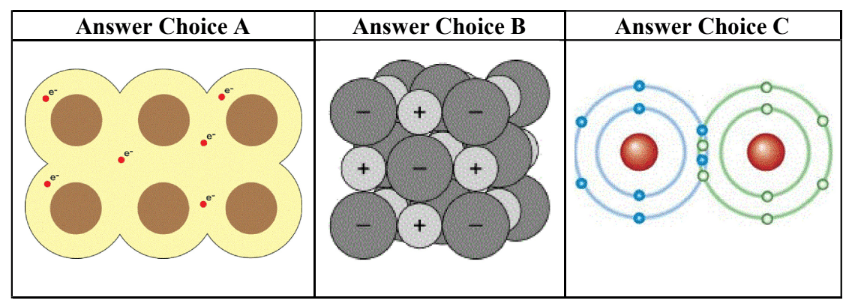
A - metallic
B - Ionic
C- Covalent
Which answer choice is an ionic bond?
NaCl H2O Fe
What is the empirical formula of CO2?
CO2
What is the shape of the molecule shown below?

linear
What is the chemical formula for diphosphorus trichloride?
P2Cl3
Ionic, Metallic, Covalent: Which of the following is brittle?
Ionic
Unknown element Z bonds with Potassium (K) in a 1:1 ratio. What is most likely unknown element Z?
any nonmetal with a -1 charge (group 17)
What is the molecular and empirical formula of the compound shown below:

Molecular: C3H6
Empirical: CH2
Is the molecule below polar or nonpolar?
nonpolar (no lone pairs)
What is the IUPAC name for P3Br5?
triphoshours pentabromide
Ionic, Metallic, Covalent: Which of the following is malleable?
Metallic
What type of bond will be formed when Magnesium (Mg) bonds to Chlorine (Cl) and what will the ratio be?
ionic bond
MgCl₂ (1:2 ratio)
Draw the lewis dot structure of N2.

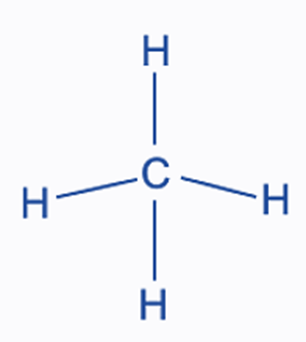
What is the molecular geometry of the compound below?
tetrahedral
What is the IUPAC name for NaF?
sodium flouride
Ionic, Metallic, Covalent: which conducts electricity as a solution (electrolyte)?
Ionic
What is the percent composition of chlorine in sodium chloride, NaCl?

60%
When drawing covalent lewis dot structures, what two elements are exceptions to the octet rule?
Hydrogen (2) and Boron (6)
Draw PH₃ and identify its molecular shape.
Trigonal Pyramidal

In the IUPAC name Iron (II) Chloride, what does the (II) represent?
Iron has a +2 ion charge; charge of cation
Which substance below is flammable?
MgO Fe O₂
O2
covalents are flammable
What is the correct formula for magnesium hydroxide?
Mg(OH)2
Draw the lewis dot structure of NH3.

What is the molecular shape of water? and is it a polar molecule?
Bent and Polar
What is the IUPAC name for Na2SO4?