Name all 3 INTRAmolecular forces from strongest to weakest. (Must get all 3 for points)
Metallic Bonding
Ionic Bonding
Covalent Bonding (can be split into polar and nonpolar, with nonpolar being the weakest)
Name all three INTERmolecularforces from strongest to weakest. (Must name all 3 for points)
Hydrogen bonding
Dipole dipole
London dispersion
Freezing lemonade is an example of a....
a) Physical Property
b) Chemical Property
c) Physical Change
d) Chemical Change
c) Physical Change
Where is the study guide key?
on eClass under "content" in "Unit 4"
When is the eClass quiz for this unit due?
Monday 10/27 at 11:59PM
(Make sure you did the right one, HG = Honors)
In a ____________ (ionic or covalent) bond, electrons are shared between ____________ (metals or nonmetals).
In a COVALENT bond, electrons are shared between NONMETALS.
What type of intermolecular force will the H2S experience?
Dipole dipole
Which of these is matter?
a) ideas
b) air
c) space
d) sound
b) air
What is the name for Te4O5? (SPELLING COUNTS!)
Tetratellurium pentaoxide
Use prefixes for covalent (nonmetal nonmetal)
What's an element in the same period as Oxygen (O)?
Li, Be, B, C, N, O, F, or Ne
In a ____________ (ionic or covalent) bond, a ___________ (metal or nonmetal) transfers electrons to a ____________ (metal or nonmetal).
MUST HAVE ALL THREE CORRECT FOR POINTS
What kind of intramolecular force is experienced in the compound CCl4?
a) ionic
b) metallic
c) polar covalent
d) nonpolar covalent
d) nonpolar covalent
A density column is created with three substances, which substance would be at the bottom? Use the data below.
Substance A: 0.4 g/cm3
Substance B: 1.5 g/cm3
Substance C: 1.0 g/cm3
Substance B: 1.5 g/cm3 (it's the most dense so it will sink to the bottom)
Review Unit 2 (Periodic Table Trends)
What is the trend for radius across a period?
Decreases as you go to the right
1. What element is 1s2 2s2 2p5?
2. What is an element that will have similar properties to this one?
(MUST ANSWER BOTH CORRECTLY)
1. Fluorine
2. Any other element in that group (Cl, Br, I, At, Ts)
Which one is polar which one is nonpolar?
A) B)
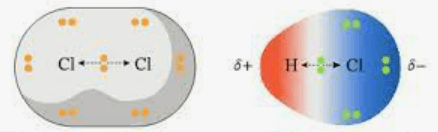
A) is nonpolar
B) is polar
Which compound is most likely to be a gas at room temperature?
a) NaCl
b) CaOH
c) H2O
d) SiCl4
d) SiCl4
Because it is nonpolar covalent so it's intermolecular force is london dispersion (the weakest one!)
Which of these is a chemical change?
a) Baking
b) Melting
c) Evaporating
d) Tearing
Baking
What is the name of F2?
Fluorine
(if it's just one element, you just put the element name)
An atom has an atomic number of 21 and a mass of 45. How many neutrons does this atom have?
24
(mass - atomic number)
What type of force is BETWEEN molecules?
a) Intramolecular
b) Intermolecular
INTERmolecular
What type of intermolecular force will be experienced by CCl4?
Dispersion forces
What is the density of a rock that has a mass of 5.09 g and when placed in a graduated cylinder, the readings are 10 ml then 17 mL.
5.09 g / 7 mL = 0.73 g/mL
What is the name of NiBr2?
Nickel (II) Bromide
A new element is discovered and has 2 valence electrons and is a metal. What group would this element fit into?
a) Alkali metals
b) Alkaline earth metals
c) Halogens
d) Noble Gases
b) Alkaline earth metals
Which forces are stronger: Intramolecular or Intermolecular?
Intramolecular (bonds)
Which compound will have the highest viscosity and boiling point?
a) HF
b) S2
c) CH3
d)CO2
a) HF
(Strongest IMF)
What is the mass of an object whose density is 4.97g/cm3 and volume is 15.25 cm3? Round to the one decimal place (0.1). REMEMBER UNITS (WRONG UNITS = NO POINTS)!
75.8 g
Review Unit 1 (Isotopes)
1. Calculate the average atomic mass for an unknown element whose isotopes have the following masses 23.985(78.99%), 24.986(10.00%), and 25.983 (11.01%).
2. What element is this?
1) 24.305 amu
2) Magnesium (Mg)
All or nothing
JJ Thompson's discovery of the (1)_____________ lead him to revise the solid sphere model and instead he created the (2)__________ _______________ model.
After JJ Thompson, Rutherford did the gold foil experiment and observed that when he shot particles at the sheet of gold foil, most of them went through it and a few of them bounced off. This lead him to conclude that the atom is mostly (3)___________ _________, but it has a (4)___________ at the center.
1) electron
2) plum pudding
3) empty space
4) nucleus