What are the three Types of Chemical Bonds?
Metallic, Covalent, and Ionic
What is a diatomic molecule? List ALL examples.
Two atoms of the same element. BrINClHOF.
What are the Lines representing in a Lewis dot structure?
These are used to represent an electron pair in a covalent bond
Which of the following atoms has the LARGEST atomic radii?
Fluorine, Cesium, Zinc, Aluminum.
Cesium!
Identify the polyatomic ion
SO3 2-
Sulfite
What type of structure would be formed from an ionic bonds?
A Crystal Lattice.
What is the tendency of atoms to attract electrons for bonding purposes called?
Electronegativity
Draw the Lewis Structure for CO2

Identify the element with the following noble gas notation:
[Xe] 6s2 4f14 5d9
Au
What type of charge does the Phosphate polyatomic ion have?
-3
Which bond transfers electrons?
Ionic bonds.
What are electrons that are involved in atomic bonding called?
Valence electrons
Draw the Lewis Structure for NH3

What's the charge of the following elements?
Mg, B, and Cl.
Mg+2
B+3
Cl-1
Write the formulas for the following polyatomic ions:
Carbonate
Perchlorate
Chromate
Carbonate: CO32-
Perchlorate ClO4-
Chromate CrO42-
What type of bond is a sea of electrons which are shared?
Metallic Bonds
What type of bond is found in HCl
(EN for H - 2.1; EN for Cl - 3.0)
3.0 - 2.1 = 0.9
Polar Covalent
How many lone pairs would each oxygen atom have in the Lewis structure for O2?
TWO!

FALSE. Anions gain electrons which makes them bigger than cations.
If we were to convert the density of 1,560 kg/L to cg/fm3, what conversion factors would we need?
1 kg = 103 g
1 cg = 10-2 g
1 mL = 10-3 L
1 mL = 1 cm3
1 cm3 = 10-6 m3
1 fm3 = 10-45 m3
What is a bond formed when atoms share electrons unequally?
Polar Covalent Bonds
List the IMFs from STRONGEST to WEAKEST.
Hydrogen Bonding > Dipole-Dipole > London-Dispersion
2 single bonds in total!
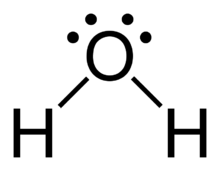
Why do atoms bond?
Atoms tend to form bonds to acquire the stability of a noble gas.
Convert 3.49 km3 to nm3.
(3.49 km3/ 1) × (109 m3/1 km3) × (1 nm3/ 10-27 m3)= 3.49 x 1036 nm3